Method 321 -
Measurement of Gaseous Hydrogen Chloride Emissions At
Portland Cement Kilns
by Fourier Transform Infrared (FTIR) Spectroscopy
1.3 Method Range and
Sensitivity.
4.1.1 Background
Interferences.
4.2 Sampling System
Interferences.
6.1 FTIR Spectrometer
and Detector.
6.3 Mass Flow
Meters/Controllers.
6.4
Polytetrafluoroethane tubing.
6.11
Calibration/Analyte Spike Assembly.
8.0 Sample Collection,
Preservation and Storage
8.4 Pre-Test
Calibration Transfer Standard (Direct Instrument Calibration).
8.5 Pre-Test System
Calibration.
8.6.3 Continuous Flow
Through Sampling.
8.8 Sampling QA, Data
Storage and Reporting.
10.0 Calibration and
Standardization
10.1 Calibration
transfer standards (CTS).
10.2 Signal-to-Noise
Ratio (S/N).
12.0 Data Analysis and
Calculations
1.0 Introduction
This method should be
performed by those persons familiar with the operation of Fourier Transform
Infrared (FTIR) instrumentation in the application to source sampling. This
document describes the sampling procedures for use in the application of FTIR
spectrometry for the determination of vapor phase hydrogen chloride (HCl)
concentrations both before and after particulate matter control devices
installed at portland cement kilns. A procedure for analyte spiking is included
for quality assurance. This method is considered to be self validating provided
that the requirements listed in section 9 of this method are followed. The
analytical procedures for interpreting infrared spectra from emission
measurements are described in the "Protocol For The Use of Extractive
Fourier Transform Infrared (FTIR) Spectrometry in Analyses of Gaseous Emissions
From Stationary Industrial Sources", included as an addendum to proposed
Method 320 of this appendix (hereafter referred to as the "FTIR
Protocol)". References 1 and 2 describe the use of FTIR spectrometry in field
measurements. Sample transport presents the principal difficulty in directly
measuring HCl emissions. This identical problem must be overcome by any
extractive measurement method. HCl is reactive and water soluble. The sampling
system must be adequately designed to prevent sample condensation in the
system.
1.1 Scope and Application
This method is
specifically designed for the application of FTIR Spectrometry in extractive
measurements of gaseous HCl concentrations in Portland cement kiln emissions.
1.2 Applicability.
This method applies to the
measurement of HCl [CAS No. 7647-01-0]. This method can be applied to the
determination of HCl concentrations both before and after particulate matter
control devices installed at Portland cement manufacturing facilities. This
method applies to either continuous flow through measurement (with isolated
sample analysis) or grab sampling (batch analysis). HCl is measured using the
mid-infrared spectral region for analysis (about 400 to 4000 cm-1 or 25 to 2.5 µm). Table 1 lists the suggested analytical region for
quantification of HCl taking the interference from water vapor into
consideration.
TABLE 1. EXAMPLE
ANALYTICAL REGION FOR HCl.

1.3 Method Range and Sensitivity.
1.3.1 The analytical range
is determined by the instrumental design and the composition of the gas stream.
For practical purposes there is no upper limit to the range because the path length
may be reduced or the sample may be diluted. The lower detection range depends
on (1) the absorption coefficient of the compound in the analytical frequency
region, (2) the spectral resolution, (3) the interferometer sampling time, (4)
the detector sensitivity and response, and (5) the absorption path length.
1.3.2 The practical lower
quantification range is usually higher than the instrument sensitivity allows
and is dependent upon (1) the presence of interfering species in the exhaust
gas including H2O, CO2, and SO2, (2) analyte losses in the sampling system, (3) the optical alignment
of the gas cell and transfer optics, and (4) the quality of the reflective
surfaces in the cell (cell throughput). Under typical test conditions (moisture
content of up to 30% and CO2 concentrations from 1 to 15
percent), a 22 meter path length cell with a suitable sampling system may
achieve a lower quantification range of from 1 to 5 ppm for HCl.
1.4 Data Quality Objectives.
1.4.1 In designing or
configuring the analytical system, data quality is determined by measuring of
the root mean square deviation (RMSD) of the absorbance values within a chosen
spectral (analytical) region. The RMSD provides an indication of the
signal-to-noise ratio (S/N) of the spectral baseline. Appendix D of the FTIR
Protocol (the addendum to Method 320 of this appendix) presents a discussion of
the relationship between the RMSD, lower detection limit, DLi, and analytical uncertainty,
AUi. It is important to consider the target analyte
quantification limit when performing testing with FTIR instrumentation, and to
optimize the system to achieve the desired detection limit.
1.4.2 Data quality is
determined by measuring the root mean square (RMS) noise level in each
analytical spectral region (appendix C of the FTIR Protocol). The RMS noise is
defined as the root mean square deviation (RMSD) of the absorbance values in an
analytical region from the mean absorbance value in the same region. Appendix D
of the FTIR Protocol defines the minimum analyte uncertainty (MAU), and how the
RMSD is used to calculate the MAU. The MAUim is the minimum
concentration of the ith analyte in the mth analytical region for which the
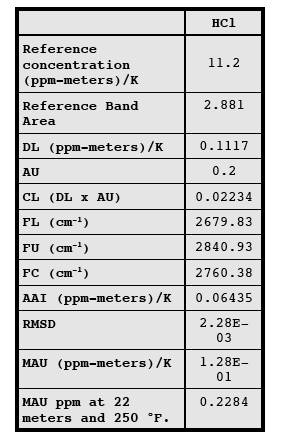
analytical uncertainty limit can be maintained. Table 2 presents example values
of AU and MAU using the analytical region presented in Table 1.
TABLE 2. EXAMPLE PRE-TEST PROTOCOL CALCULATIONS
FOR HYDROGEN CHLORIDE
2.0 Summary of Method
2.1 Principle.
See Method 320 of this
appendix. HCl can also undergo rotation transitions by absorbing energy in the
far-infrared spectral region. The rotational transitions are superimposed on
the vibrational fundamental to give a series of lines centered at the
fundamental vibrational frequency, 2885 cm-1. The frequencies
of absorbance and the pattern of rotational/vibrational lines are unique to
HCl. When this distinct pattern is observed in an infrared spectrum of an unknown
sample, it unequivocally identifies HCl as a component of the mixture. The
infrared spectrum of HCl is very distinctive and cannot be confused with the
spectrum of any other compound. See Reference 6.
2.2 Sampling and Analysis.
See Method 320 of this
appendix.
2.3 Operator Requirements.
The analyst must have
knowledge of spectral patterns to choose an appropriate absorption path length
or determine if sample dilution is necessary. The analyst should also
understand FTIR instrument operation well enough to choose instrument settings
that are consistent with the objectives of the analysis.
3.0 Definitions
See appendix A of the FTIR
Protocol.
4.0 Interferences
This method will not
measure HCl under conditions: (1) where the sample gas stream can condense in
the sampling system or the instrumentation, or (2) where a high moisture
content sample relative to the analyte concentrations imparts spectral
interference due to the water vapor absorbance bands. For measuring HCl the
first (sampling) consideration is more critical. Spectral interference from
water vapor is not a significant problem except at very high moisture levels
and low HCl concentrations.
4.1 Analytical Interferences.
See Method 320 of this
appendix.
4.1.1 Background Interferences.
See Method 320 of this
appendix.
4.1.2 Spectral interferences.
Water vapor can present
spectral interference for FTIR gas analysis of HCl. Therefore, the water vapor
in the spectra of kiln gas samples must be accounted for. This means preparing
at least one spectrum of a water vapor sample where the moisture concentration
is close to that in the kiln gas.
4.2 Sampling System Interferences.
The principal sampling
system interferant for measuring HCl is water vapor. Steps must be taken to
ensure that no condensation forms anywhere in the probe assembly, sample lines,
or analytical instrumentation. Cold spots anywhere in the sampling system must
be avoided. The extent of sampling system bias in the FTIR analysis of HCl
depends on concentrations of potential interferants, moisture content of the
gas stream, temperature of the gas stream, temperature of sampling system
components, sample flow rate, and reactivity of HCl with other species in the
gas stream (e.g., ammonia). For measuring HCl in a wet gas stream the
temperatures of the gas stream, sampling components, and the sample flow rate
are of primary importance. Analyte spiking with HCl is performed to demonstrate
the integrity of the sampling system for transporting HCl vapor in the flue gas
to the FTIR instrument. See section 9 of this method for a complete description
of analyte spiking.
5.0 Safety
5.1 Hydrogen chloride
vapor is corrosive and can cause irritation or severe damage to respiratory
system, eyes and skin. Exposure to this compound should be avoided.
5.2 This method may
involve sampling at locations having high positive or negative pressures, or
high concentrations of hazardous or toxic pollutants, and can not address all
safety problems encountered under these diverse sampling conditions. It is the
responsibility of the tester(s) to ensure proper safety and health practices,
and to determine the applicability of regulatory limitations before performing
this test method. Leak-check procedures are outlined in section 8.2 of Method
320 of this appendix.
6.0 Equipment and Supplies
Note: Mention of trade
names or specific products does not constitute endorsement by the Environmental
Protection Agency.
6.1 FTIR Spectrometer and Detector.
An FTIR Spectrometer
system (interferometer, transfer optics, gas cell and detector) having the
capability of measuring HCl to the predetermined minimum detectable level
required (see section 4.1.3 of the FTIR Protocol). The system must also include
an accurate means to control and/or measure the temperature of the FTIR gas
analysis cell, and a personal computer with compatible software that provides
real-time updates of the spectral profile during sample and spectral
collection.
6.2 Pump.
Capable of evacuating the
FTIR cell volume to 1 Torr (133.3 Pascals) within two minutes (for batch sample
analysis).
6.3 Mass Flow Meters/Controllers.
To accurately measure
analyte spike flow rate, having the appropriate calibrated range and a stated
accuracy of ± 2 percent of the absolute measurement value. This device must be
calibrated with the major component of the calibration/spike gas (e.g.,
nitrogen) using an NIST traceable bubble meter or equivalent. Single point
calibration checks should be performed daily in the field. When spiking HCl,
the mass flow meter/controller should be thoroughly purged before and after
introduction of the gas to prevent corrosion of the interior parts.
6.4 Polytetrafluoroethane tubing.
Diameter and length
suitable to connect cylinder regulators.
6.5 Stainless Steel tubing.
Type 316 of appropriate
length and diameter for heated connections.
6.6 Gas Regulators.
Purgeable HCl regulator.
6.7 Pressure Gauge.
Capable of measuring
pressure from 0 to 1000 Torr (133.3 Pa=1 Torr) within ± 5 percent.
6.8 Sampling Probe.
Glass, stainless steel or
other appropriate material of sufficient length and physical integrity to
sustain heating, prevent adsorption of analytes and capable of reaching gas
sampling point.
6.9 Sampling Line.
Heated 180 ¼C (360 ¼F) and
fabricated of either stainless steel, polytetrafluoroethane or other material
that prevents adsorption of HCl and transports effluent to analytical
instrumentation. The extractive sample line must have the capability to
transport sample gas to the analytical components as well as direct heated
calibration spike gas to the calibration assembly located at the sample probe.
It is important to minimize the length of heated sample line.
6.10 Particulate Filters.
A sintered stainless steel
filter rated at 20 microns or greater may be placed at the inlet of the probe
(for removal of large particulate matter). A heated filter (Balston¨ or equivalent) rated at 1 micron is necessary for primary particulate
matter removal, and shall be placed immediately after the heated probe. The
filter/filter holder temperature should be maintained at 180 ¼C (360 ¼F).
6.11 Calibration/Analyte Spike Assembly.
A heated three12 way valve
assembly (or equivalent) to introduce surrogate spikes into the sampling system
at the outlet of the probe before the primary particulate filter.
6.12 Sample Extraction Pump.
A leak-free heated head
pump (KNF¨ Neuberger or equivalent) capable of extracting sample
effluent through entire sampling system at a rate which prevents analyte losses
and minimizes analyzer response time. The pump should have a heated by-pass and
may be placed either before the FTIR instrument or after. If the sample pump is
located upstream of the FTIR instrument, it must be fabricated from materials
non-reactive to HCl. The sampling system and FTIR measurement system shall
allow the operator to obtain at least six sample spectra during a one-hour
period.
6.13 Barometer.
For measurement of
barometric pressure.
6.14 Gas Sample Manifold.
A distribution manifold
having the capabilities listed in sections 6.14.1 through 6.14.4;
6.14.1 Delivery of
calibration gas directly to the analytical instrumentation;
6.14.2 Delivery of
calibration gas to the sample probe (system calibration or analyte spike) via a
heated traced sample line;
6.14.3 Delivery of sample
gas (kiln gas, spiked kiln gas, or system calibrations) to the analytical
instrumentation;
6.14.4 Delivery (optional)
of a humidified nitrogen sample stream.
6.15 Flow Measurement Device.
Type S Pitot tube (or
equivalent) and Magnahelic¨
set for measurement of volumetric flow
rate.
7.0 Reagents and Standards
HCl can be purchased in a
standard compressed gas cylinder. The most stable HCl cylinder mixture
available has a concentration certified at ±5 percent. Such a cylinder is
suitable for performing analyte spiking because it will provide reproducible
samples. The stability of the cylinder can be monitored over time by
periodically performing direct FTIR analysis of cylinder samples. It is
recommended that a 10-50 ppm cylinder of HCl be prepared having from 2-5 ppm
SF6 as a tracer compound. (See sections 7.1 through 7.3 of Method 320 of this
appendix for a complete description of the use of existing HCl reference
spectra. See section 9.1 of Method 320 of this appendix for a complete
discussion of standard concentration selection.)
8.0 Sample Collection, Preservation and Storage
See also Method 320 of
this appendix.
8.1 Pretest.
A screening test is ideal
for obtaining proper data that can be used for preparing analytical program
files. Information from literature surveys and source personnel is also
acceptable. Information about the sampling location and gas stream composition
is required to determine the optimum sampling system configuration for
measuring HCl. Determine the percent moisture of the kiln gas by Method 4 of
appendix A to part 60 of this chapter or by performing a wet bulb/dry bulb
measurement. Perform a preliminary traverse of the sample duct or stack and
select the sampling point(s). Acquire an initial spectrum and determine the
optimum operational path length of the instrument.
8.2 Leak-Check.
See Method 320 of this
appendix, section 8.2 for direction on performing leak-checks.
8.3 Background Spectrum.
See Method 320 of this
appendix, section 8.5 for direction in background spectral acquisition.
8.4 Pre-Test Calibration Transfer Standard (Direct Instrument Calibration).
See Method 320 of this
appendix, section 8.3 for direction in CTS spectral acquisition.
8.5 Pre-Test System Calibration.
See Method 320 of this
appendix, sections 8.6.1 through 8.6.2 for direction in performing system
calibration.
8.6 Sampling.
8.6.1 Extractive System.
An extractive system
maintained at 180 ¼C (360 ¼F) or higher which is capable of directing a total
flow of at least 12 L/min to the sample cell is required (References 1 and 2).
Insert the probe into the duct or stack at a point representing the average
volumetric flow rate and 25
percent of the cross sectional area. Co-locate an appropriate flow monitoring
device with the sample probe so that the flow rate is recorded at specified
time intervals during emission testing (e.g., differential pressure
measurements taken every 10 minutes during each run).
8.6.2 Batch Samples.
Evacuate the absorbance
cell to 5 Torr (or less) absolute pressure before taking first sample. Fill the
cell with kiln gas to ambient pressure and record the infrared spectrum, then
evacuate the cell until there is no further evidence of infrared absorption.
Repeat this procedure, collecting a total of six separate sample spectra within
a 1-hour period.
8.6.3 Continuous Flow Through Sampling.
Purge the FTIR cell with
kiln gas for a time period sufficient to equilibrate the entire sampling system
and FTIR gas cell. The time required is a function of the mechanical response
time of the system (determined by performing the system calibration with the
CTS gas or equivalent), and by the chemical reactivity of the target analytes.
If the effluent target analyte concentration is not variable, observation of
the spectral up-date of the flowing gas sample should be performed until
equilibration of the sample is achieved. Isolate the gas cell from the sample
flow by directing the purge flow to vent. Record the spectrum and pressure of
the sample gas. After spectral acquisition, allow the sample gas to purge the
cell with at least three volumes of kiln gas. The time required to adequately
purge the cell with the required volume of gas is a function of 1) cell volume,
2) flow rate through the cell, and 3) cell design. It is important that the gas
introduction and vent for the FTIR cell provides a complete purge through the
cell.
8.6.4 Continuous Sampling.
In some cases it is
possible to collect spectra continuously while the FTIR cell is purged with
sample gas. The sample integration time, tss, the sample flow
rate through the gas cell, and the sample integration time must be chosen so
that the collected data consist of at least 10 spectra with each spectrum being
of a separate cell volume of flue gas. Sampling in this manner may only be
performed if the native source analyte concentrations do not affect the test
results.
8.7 Sample Conditioning
8.7.1 High Moisture Sampling.
Kiln gas emitted from wet
process cement kilns may contain 3- to 40 percent moisture. Zinc selenide
windows or the equivalent should be used when attempting to analyze hot/wet
kiln gas under these conditions to prevent dissolution of water soluble window
materials (e.g., KBr).
8.7.2 Sample Dilution.
The sample may be diluted
using an in-stack dilution probe, or an external dilution device provided that
the sample is not diluted below the instrument's quantification range. As an
alternative to using a dilution probe, nitrogen may be dynamically spiked into
the effluent stream in the same manner as analyte spiking. A constant dilution
rate shall be maintained throughout the measurement process. It is critical to
measure and verify the exact dilution ratio when using a dilution probe or the
nitrogen spiking approach. Calibrating the system with a calibration gas
containing an appropriate tracer compound will allow determination of the
dilution ratio for most measurement systems. The tester shall specify the
procedures used to determine the dilution ratio, and include these calibration
results in the report.
8.8 Sampling QA, Data Storage and Reporting.
See the FTIR Protocol.
Sample integration times shall be sufficient to achieve the required
signal-to-noise ratio, and all sample spectra should have unique file names.
Two copies of sample interferograms and processed spectra will be stored on
separate computer media. For each sample spectrum the analyst must document the
sampling conditions, the sampling time (while the cell was being filled), the
time the spectrum was recorded, the instrumental conditions (path length,
temperature, pressure, resolution, integration time), and the spectral file
name. A hard copy of these data must be maintained until the test results are
accepted.
8.9 Signal Transmittance.
Monitor the signal
transmittance through the instrumental system. If signal transmittance
(relative to the background) drops below 95 percent in any spectral region
where the sample does not absorb infrared energy, then a new background
spectrum must be obtained.
8.10 Post-test CTS.
After the sampling run
completion, record the CTS spectrum. Analysis of the spectral band area used
for quantification from pre- and post-test CTS spectra should agree to within
±5 percent or corrective action must be taken.
8.11 Post-test QA.
The sample spectra shall
be inspected immediately after the run to verify that the gas matrix
composition was close to the assumed gas matrix, (this is necessary to account
for the concentrations of the interferants for use in the analytical analysis
programs), and to confirm that the sampling and instrumental parameters were
appropriate for the conditions encountered.
9.0 Quality Control
Use analyte spiking to
verify the effectiveness of the sampling system for the target compounds in the
actual kiln gas matrix. QA spiking shall be performed before and after each
sample run. QA spiking shall be performed after the pre- and post-test CTS
direct and system calibrations. The system biases calculated from the pre- and
post-test dynamic analyte spiking shall be within ±30 percent for the spiked
surrogate analytes for the measurements to be considered valid. See sections
9.3.1 through 9.3.2 for the requisite calculations. Measurement of the
undiluted spike (direct-to-cell measurement) involves sending dry, spike gas to
the FTIR cell, filling the cell to 1 atmosphere and obtaining the spectrum of
this sample. The direct-to-cell measurement should be performed before each
analyte spike so that the recovery of the dynamically spiked analytes may be
calculated. Analyte spiking is only effective for assessing the integrity of
the sampling system when the concentration of HCl in the source does not vary
substantially. Any attempt to quantify an analyte recovery in a variable
concentration matrix will result in errors in the expected concentration of the
spiked sample. If the kiln gas target analyte concentrations vary by more than
±5 percent (or 5 ppm, whichever is greater) in the time required to acquire a
sample spectrum, it may be necessary to: 1) use a dual sample probe approach,
2) use two independent FTIR measurement systems, 3) use alternate QA/QC
procedures, or 4) postpone testing until stable emission concentrations are
achieved. (See section 9.2.3 of this method). It is recommended that a
laboratory evaluation be performed before attempting to employ this method under
actual field conditions. The laboratory evaluation shall include 1) performance
of all applicable calculations in section 4 of the FTIR Protocol; 2) simulated
analyte spiking experiments in dry (ambient) and humidified sample matrices
using HCl; and 3) performance of bias (recovery) calculations from analyte
spiking experiments. It is not necessary to perform a laboratory evaluation
before every field test. The purpose of the laboratory study is to demonstrate
that the actual instrument and sampling system configuration used in field
testing meets the requirements set forth in this method.
9.1 Spike Materials.
Perform analyte spiking
with an HCl standard to demonstrate the integrity of the sampling system.
9.1.1 An HCl standard of
approximately 50 ppm in a balance of ultra pure nitrogen is recommended. The SF6 (tracer) concentration shall be 2 to 5 ppm depending upon the
measurement path length. The spike ratio (spike flow/total flow) shall be no
greater than 1:10, and an ideal spike concentration should approximate the
native effluent concentration.
9.1.2 The ideal spike
concentration may not be achieved because the target concentration cannot be
accurately predicted prior to the field test, and limited calibration standards
will be available during testing. Therefore, practical constraints must be
applied that allow the tester to spike at an anticipated concentration. For
these tests, the analyte concentration contributed by the HCl standard spike
should be 1 to 5 ppm or should more closely approximate the native
concentration if it is greater.
9.2 Spike Procedure
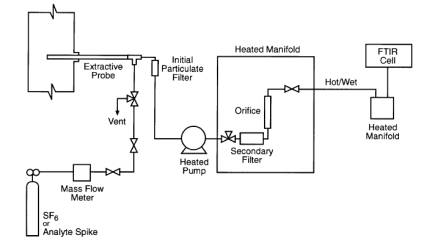
9.2.1 A spiking/sampling
apparatus is shown in Figure 2. Introduce the spike/tracer gas mixture at a
constant flow (±2 percent) rate at approximately 10 percent of the total sample
flow. (For example, introduce the surrogate spike at 1 L/min ± 20 cc/min, into
a total sample flow rate of 10 L/min). The spike must be pre-heated before
introduction into the sample matrix to prevent a localized condensation of the
gas stream at the spike introduction point. A heated sample transport line(s)
containing multiple transport tubes within the heated bundle may be used to
spike gas up through the sampling system to the spike introduction point. Use a
calibrated flow device (e.g., mass flow meter/controller), to monitor the spike
flow as indicated by a calibrated flow meter or controller, or alternately, the
SF6 tracer ratio may be calculated from the direct
measurement and the diluted measurement. It is often desirable to use the
tracer approach in calculating the spike/total flow ratio because of the
difficulty in accurately measuring hot/wet total flow. The tracer technique has
been successfully used in past validation efforts (Reference 1).
9.2.2 Perform a
direct-to-cell measurement of the dry, undiluted spike gas. Introduce the spike
directly to the FTIR cell, bypassing the sampling system. Fill cell to 1
atmosphere and collect the spectrum of this sample. Ensure that the spike gas
has equilibrated to the temperature of the measurement cell before acquisition
of the spectra. Inspect the spectrum and verify that the gas is dry and
contains negligible CO2. Repeat the process to obtain a second
direct-to-cell measurement. Analysis of spectral band areas for HCl from these
duplicate measurements should agree to within ± 5 percent of the mean.
9.2.3 Analyte Spiking.
Determine whether the kiln gas contains native concentrations of HCl by
examination of preliminary spectra. Determine whether the concentration varies
significantly with time by observing a continuously up-dated spectrum of sample
gas in the flow-through sampling mode. If the concentration varies by more than
± 5 percent during the period of time required to acquire a spectra, then an
alternate approach should be used. One alternate approach uses two sampling
lines to convey sample to the gas distribution manifold. One of the sample
lines is used to continuously extract unspiked kiln gas from the source. The
other sample line serves as the analyte spike line. One FTIR system can be used
in this arrangement. Spiked or unspiked sample gas may be directed to the FTIR
system from the gas distribution manifold, with the need to purge only the
components between the manifold and the FTIR system. This approach minimizes
the time required to acquire an equilibrated sample of spiked or unspiked kiln
gas. If the source varies by more than ± 5 percent (or 5 ppm, whichever is
greater) in the time it takes to switch from the unspiked sample line to the
spiked sample line, then analyte spiking may not be a feasible means to
determine the effectiveness of the sampling system for the HCl in the sample
matrix. A second alternative is to use two completely independent FTIR
measurement systems. One system would measure unspiked samples while the other
system would measure the spiked samples. As a last option, (where no other
alternatives can be used) a humidified nitrogen stream may be generated in the
field which approximates the moisture content of the kiln gas. Analyte spiking
into this humidified stream can be employed to assure that the sampling system
is adequate for transporting the HCl to the FTIR instrumentation.
9.2.3.1 Adjust the spike
flow rate to approximately 10 percent of the total flow by metering spike gas
through a calibrated mass flowmeter or controller. Allow spike flow to
equilibrate within the sampling system before analyzing the first spiked kiln
gas samples. A minimum of two consecutive spikes are required. Analysis of the
spectral band area used for quantification should agree to within ± 5 percent
or corrective action must be taken.
9.2.3.2 After QA spiking
is completed, the sampling system components shall be purged with nitrogen or
dry air to eliminate traces of the HCl compound from the sampling system
components. Acquire a sample spectra of the nitrogen purge to verify the
absence of the calibration mixture.
9.2.3.3 Analyte spiking
procedures must be carefully executed to ensure that meaningful measurements
are achieved. The requirements of sections 9.2.3.3.1 through 9.2.3.3.4 shall be
met.
9.2.3.3.1 The spike must
be in the vapor phase, dry, and heated to (or above) the kiln gas temperature
before it is introduced to the kiln gas stream.
9.2.3.3.2 The spike flow
rate must be constant and accurately measured.
9.2.3.3.3 The total flow
must also be measured continuously and reliably or the dilution ratio must
otherwise be verified before and after a run by introducing a spike of a
non-reactive, stable compound (i.e., tracer).
9.2.3.3.4 The tracer must
be inert to the sampling system components, not contained in the effluent gas,
and readily detected by the analytical instrumentation. Sulfur hexafluoride (SF6) has been used successfully (References 1 and 2) for this purpose.
9.3 Calculations
9.3.1 Recovery.
Calculate the percent
recovery of the spiked analytes using equations 1 and 2.
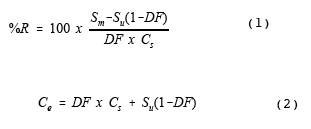
The spike dilution factor
may be confirmed by measuring the total flow and the spike flow directly.
Alternately, the spike dilution can be verified by comparing the concentration
of the tracer compound in the spiked samples (diluted) to the tracer
concentration in the direct (undiluted) measurement of the spike gas.
If SF6 is the tracer gas, then

9.3.2 Bias.
The bias may be determined
by the difference between the observed spike value and the expected response
(i.e., the equivalent concentration of the spiked material plus the analyte
concentration adjusted for spike dilution). Bias is defined by section 6.3.1 of
EPA Method 301 of this appendix (Reference 8) as,
![]()
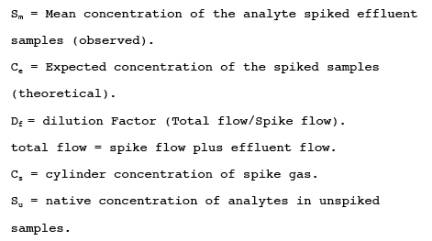
where:
B = Bias at spike level.
Sm = Mean concentration of the analyte spiked samples.
Ce = Expected concentration of the analyte in spiked samples.
Acceptable recoveries for
analyte spiking are ± 30 percent. Application of correction factors to the data
based upon bias and recovery calculations is subject to the approval of the
Administrator.
10.0 Calibration and Standardization
10.1 Calibration transfer standards (CTS).
The EPA Traceability
Protocol gases or NIST traceable standards, with a minimum accuracy of ± 2
percent shall be used. For other requirements of the CTS, see the FTIR Protocol
section 4.5.
10.2 Signal-to-Noise Ratio (S/N).
The S/N shall be less than
the minimum acceptable measurement uncertainty in the analytical regions to be
used for measuring HCl.
10.3 Absorbance Path length.
Verify the absorbance path
length by comparing CTS spectra to reference spectra of the calibration
gas(es).
10.4 Instrument Resolution.
Measure the line width of
appropriate CTS band(s) to verify instrumental resolution.
10.5 Apodization Function.
Choose the appropriate
apodization function. Determine any appropriate mathematical transformations
that are required to correct instrumental errors by measuring the CTS. Any
mathematical transformations must be documented and reproducible. Reference 9
provides additional information about FTIR instrumentation.
11.0 Analytical Procedure
A full description of the
analytical procedures is given in sections 4.6 - 4.11, sections 5, 6, and 7,
and the appendices of the FTIR Protocol. Additional description of quantitative
spectral analysis is provided in References 10 and 11.
12.0 Data Analysis and Calculations
Data analysis is performed
using appropriate reference spectra whose concentrations can be verified using
CTS spectra. Various analytical programs (References 10 and 11) are available
to relate sample absorbance to a concentration standard. Calculated
concentrations should be verified by analyzing spectral baselines after
mathematically subtracting scaled reference spectra from the sample spectra. A
full description of the data analysis and calculations may be found in the FTIR
Protocol (sections 4.0, 5.0, 6.0 and appendices).
12.1 Calculated
concentrations in sample spectra are corrected for differences in absorption
path length between the reference and sample spectra by
![]()
where:
Ccorr = The path length corrected concentration.
Ccalc = The initial calculated concentration (output of the multicomponent
analysis program designed for the compound).
Lr = The path length associated with the reference spectra.
Ls = The path length associated with the sample spectra.
Ts = The absolute temperature (K) of the sample gas.
Tr = The absolute temperature (K) at which reference.
spectra were recorded.
12.2 The temperature
correction in equation 5 is a volumetric correction. It does not account for
temperature dependence of rotational-vibrational relative line intensities.
Whenever possible, the reference spectra used in the analysis should be
collected at a temperature near the temperature of the FTIR cell used in the
test to minimize the calculated error in the measurement (FTIR Protocol,
appendix D). Additionally, the analytical region chosen for the analysis should
be sufficiently broad to minimize errors caused by small differences in
relative line intensities between reference spectra and the sample spectra.
13.0 Method Performance
A description of the
method performance may be found in the FTIR Protocol. This method is self
validating provided the results meet the performance specification of the QA spike
in sections 9.0 through 9.3 of this method.
14.0 Pollution Prevention
This is a gas phase
measurement. Gas is extracted from the source, analyzed by the instrumentation,
and discharged through the instrument vent.
15.0 Waste Management
Gas standards of HCl are
handled according to the instructions enclosed with the material safety data
sheet.
16.0 References
1. "Laboratory and
Field Evaluation of a Methodology for Determination of Hydrogen Chloride
Emissions From Municipal and Hazardous Waste Incinerators," S. C.
Steinsberger and J. H. Margeson. Prepared for U.S. Environmental Protection
Agency, Research Triangle Park, NC. NTIS Report No. PB89-220586. (1989).
2. "Evaluation of HCl
Measurement Techniques at Municipal and Hazardous Waste Incinerators," S.
A. Shanklin, S. C. Steinsberger, and L. Cone, Entropy, Inc. Prepared for U.S.
Environmental Protection Agency, Research Triangle Park, NC. NTIS Report No.
PB90-221896. (1989).
3. "Fourier Transform
Infrared (FTIR) Method Validation at a Coal Fired-Boiler," Entropy, Inc.
Prepared for U.S. Environmental Protection Agency, Research Triangle Park, NC.
EPA Publication No. EPA-454/R95-004. NTIS Report No. PB95-193199. (1993).
4. "Field Validation
Test Using Fourier Transform Infrared (FTIR) Spectrometry To Measure
Formaldehyde, Phenol and Methanol at a Wool Fiberglass Production
Facility." Draft. U.S. Environmental Protection Agency Report, Entropy,
Inc., EPA Contract No. 68D20163, Work Assignment I-32.
5. Kinner, L.L., Geyer,
T.G., Plummer, G.W., Dunder, T.A., Entropy, Inc. "Application of FTIR as a
Continuous Emission Monitoring System." Presentation at 1994 International
Incineration Conference, Houston, Tx. May 10, 1994.
6. "Molecular
Vibrations; The Theory of Infrared and Raman Vibrational Spectra," E.
Bright Wilson, J. C. Decius, and P. C. Cross, Dover Publications, Inc., 1980.
For a less intensive treatment of molecular rotational-vibrational spectra see,
for example, "Physical Chemistry," G. M. Barrow, chapters 12, 13, and
14, McGraw Hill, Inc., 1979.
7. "Laboratory and
Field Evaluations of Ammonium Chloride Interference in Method 26," U.S.
Environmental Protection Agency Report, Entropy, Inc., EPA Contract No.
68D20163, Work Assignment No. I-45.
8. 40 CFR 63, appendix A.
Method 301 - Field Validation of Pollutant Measurement Methods from Various
Waste Media.
9. "Fourier Transform
Infrared Spectrometry," Peter R. Griffiths and James de Haseth, Chemical
Analysis, 83, 16-25,(1986), P. J. Elving, J. D. Winefordner and I. M. Kolthoff
(ed.), John Wiley and Sons.
10.
"Computer-Assisted Quantitative Infrared Spectroscopy," Gregory L.
McClure (ed.), ASTM Special Publication 934 (ASTM), 1987.
11. "Multivariate
Least-Squares Methods Applied to the Quantitative Spectral Analysis of
Multicomponent Mixtures," Applied Spectroscopy, 39(10), 73-84, 1985.
Figure 1. FTIR Spectra
of HCl and Water.
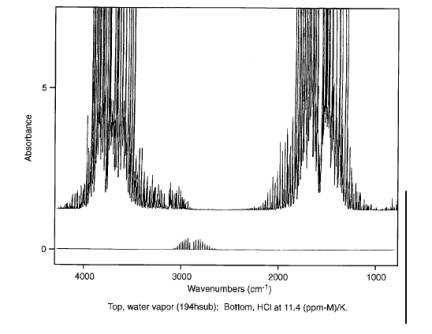
Figure 2. FTIR
Sampling/Spiking System.