METHOD 29 -
DETERMINATION OF METALS EMISSIONS FROM STATIONARY SOURCES
NOTE: This method does not include all of the
specifications (e.g. equipment
and supplies) and procedures (e.g.,
sampling and analytical) essential to its performance. Some material is
incorporated by reference from other methods in this part. Therefore, to obtain
reliable results, persons using this method should have a thorough knowledge of
at least the following additional test methods: Method
5 and Method 12.
6.3 Sample Preparation
and Analysis.
7.1 Conform to the
Specifications
7.3 Pretest Preparation
of Sampling Reagents.
7.4 Glassware Cleaning
Reagents.
7.5 Sample Digestion
and Analysis Reagents.
8.0 Sample Collection,
Preservation, Transport, and Storage.
9.1 Field Reagent
Blanks, if analyzed.
10.0 Calibration and
Standardization.
10.1 Sampling Train
Calibration.
10.2 Inductively
Coupled Argon Plasma Spectrometer Calibration.
10.3 Atomic Absorption
Spectrometer Ð Direct Aspiration AAS, GFAAS, and CVAAS analyses.
11.1.1 ICAP and ICP-MS
Analysis.
11.1.2 AAS by Direct
Aspiration and/or GFAAS.
12.0 Data Analysis and
Calculations.
13.2 Analytical
Detection Limits.
13.3 In-stack Detection
Limits.
14.0 Pollution
Prevention. [Reserved]
15.0 Waste Management.
[Reserved]
17.0 Tables, Diagrams,
Flowcharts, and Validation Data.
1.0 Scope and Application.
1.1 Analytes.
Analyte CAS
No.
Antimony (Sb) 7440-36-0
Arsenic (As) 7440-38-2
Barium (Ba) 7440-39-3
Beryllium (Be) 7440-41-7
Cadmium (Cd) 7440-43-9
Chromium (Cr) 7440-47-3
Cobalt (Co) 7440-48-4
Copper (Cu) 7440-50-8
Lead (Pb) 7439-92-1
Manganese (Mn) 7439-96-5
Mercury (Hg) 7439-97-6
Nickel (Ni) 7440-02-0
Phosphorus (P) 7723-14-0
Selenium (Se) 7782-49-2
Silver (Ag) 7440-22-4
Thallium (Tl) 7440-28-0
Zinc (Zn) 7440-66-6
1.2 Applicability.
This method is
applicable to the determination of metals emissions from stationary sources.
This method may be used to determine particulate emissions in addition to the
metals emissions if the prescribed procedures and precautions are followed.
1.2.1 Hg emissions
can be measured, alternatively, using EPA Method 101A of Appendix B, 40 CFR
Part 61. Method 101-A measures only Hg but it can be of special interest to
2.0 Summary of Method.
2.1 Principle.
A stack sample is
withdrawn isokinetically from the source, particulate emissions are collected
in the probe and on a heated filter, and gaseous emissions are then collected
in an aqueous acidic solution of hydrogen peroxide (analyzed for all metals
including Hg) and an aqueous acidic solution of potassium permanganate
(analyzed only for Hg). The recovered samples are digested, and appropriate
fractions are analyzed for Hg by cold vapor atomic absorption spectroscopy
(CVAAS) and for Sb, As, Ba, Be, Cd, Cr, Co, Cu, Pb, Mn, Ni, P, Se, Ag, Tl, and
Zn by inductively coupled argon plasma emission spectroscopy (ICAP) or atomic
absorption spectroscopy (AAS). Graphite furnace atomic absorption spectroscopy
(GFAAS) is used for analysis of Sb, As, Cd, Co, Pb, Se, and Tl if these
elements require greater analytical sensitivity than can be obtained by ICAP.
If one so chooses, AAS may be used for analysis of all listed metals if the
resulting in-stack method detection limits meet the goal of the testing
program. Similarly, inductively coupled plasma-mass spectroscopy (ICP-MS) may
be used for analysis of Sb, As, Ba, Be, Cd, Cr, Co, Cu, Pb, Mn, Ni, Ag, Tl and
Zn.
3.0 Definitions. [Reserved]
4.0 Interferences.
4.1 Iron (Fe) can be
a spectral interference during the analysis of As, Cr, and Cd by ICAP. Aluminum
(Al) can be a spectral interference during the analysis of As and Pb by ICAP.
Generally, these interferences can be reduced by diluting the analytical
sample, but such dilution raises the in-stack detection limits. Background and
overlap corrections may be used to adjust for spectral interferences. Refer to
Method 6010 of Reference 2 in Section 16.0 or the other analytical methods used
for details on potential interferences to this method. For all GFAAS analyses,
use matrix modifiers to limit interferences, and matrix match all standards.
5.0 Safety.
5.1 Disclaimer.
This method may
involve hazardous materials, operations, and equipment. This test method may
not address all of the safety problems associated with its use. It is the
responsibility of the user of this test method to establish appropriate safety
and health practices and to determine the applicability of regulatory limitations
prior to performing this test method.
5.2 Corrosive Reagents.
The following
reagents are hazardous. Personal protective equipment and safe procedures are
useful in preventing chemical splashes. If contact occurs, immediately flush
with copious amounts of water at least 15 minutes. Remove clothing under shower
and decontaminate. Treat residual chemical burn as thermal burn.
5.2.1 Nitric Acid
(HNO3). Highly corrosive to eyes, skin, nose, and
lungs. Vapors cause bronchitis, pneumonia, or edema of lungs. Reaction to
inhalation may be delayed as long as 30 hours and still be fatal. Provide
ventilation to limit exposure. Strong oxidizer. Hazardous reaction may occur
with organic materials such as solvents.
5.2.2 Sulfuric Acid
(H2SO4). Rapidly
destructive to body tissue. Will cause third degree burns. Eye damage may
result in blindness. Inhalation may be fatal from spasm of the larynx, usually
within 30 minutes. May cause lung tissue damage with edema. 1 mg/m3 for 8 hours will cause lung damage or, in higher concentrations,
death. Provide ventilation to limit inhalation. Reacts violently with metals
and organics.
5.2.3 Hydrochloric
Acid (HCl). Highly corrosive liquid with toxic vapors. Vapors are highly
irritating to eyes, skin, nose, and lungs, causing severe damage. May cause
bronchitis, pneumonia, or edema of lungs. Exposure to concentrations of 0.13 to
0.2 percent can be lethal to humans in a few minutes. Provide ventilation to
limit exposure. Reacts with metals, producing hydrogen gas.
5.2.4 Hydrofluoric
Acid (HF). Highly corrosive to eyes, skin, nose, throat, and lungs. Reaction to
exposure may be delayed by 24 hours or more. Provide ventilation to limit
exposure.
5.2.5 Hydrogen
Peroxide (H2O2). Irritating to eyes,
skin, nose, and lungs. 30% H2O2 is a strong oxidizing agent. Avoid contact with skin, eyes, and
combustible material. Wear gloves when handling.
5.2.6 Potassium
Permanganate (KMnO4). Caustic, strong oxidizer. Avoid bodily
contact with.
5.2.7 Potassium
Persulfate. Strong oxidizer. Avoid bodily contact with. Keep containers well
closed and in a cool place.
5.3 Reaction Pressure.
Due to the potential
reaction of the potassium permanganate with the acid, there could be pressure
buildup in the acidic KMnO4
absorbing solution storage bottle.
Therefore these bottles shall not be fully filled and shall be vented to
relieve excess pressure and prevent explosion potentials. Venting is required,
but not in a manner that will allow contamination of the solution. A No. 70-72
hole drilled in the container cap and Teflon liner has been used.
6.0 Equipment and Supplies.
6.1 Sampling.
A schematic of the
sampling train is shown in Figure 29-1. It has general
similarities to the Method 5 train.
6.1.1 Probe Nozzle
(Probe Tip) and Borosilicate or Quartz Glass Probe Liner. Same as Method 5,
Sections
6.1.2 Pitot Tube and
Differential Pressure Gauge. Same as Method 2,
Sections 6.1 and 6.2, respectively.
6.1.3 Filter Holder.
Glass, same as Method 5, Section 6.1.1.5,
except use a Teflon filter support or other non-metallic, non-contaminating
support in place of the glass frit.
6.1.4 Filter Heating
System. Same as Method 5, Section 6.1.1.6.
6.1.5 Condenser. Use
the following system for condensing and collecting gaseous metals and
determining the moisture content of the stack gas. The condensing system shall
consist of four to seven impingers connected in series with leak-free ground
glass fittings or other leak-free, non-contaminating fittings. Use the first
impinger as a moisture trap. The second impinger (which is the first HNO3/H2O2 impinger) shall be
identical to the first impinger in Method 5. The third impinger (which is the
second HNO3/H2O2 impinger) shall be a Greenburg Smith impinger with the standard tip
as described for the second impinger in Method 5, Section 6.1.1.8. The fourth
(empty) impinger and the fifth and sixth (both acidified KMnO4) impingers are the same as the first impinger in Method 5. Place a
temperature sensor capable of measuring to within 1ûC (2ûF) at the outlet of
the last impinger. If no Hg analysis is planned, then the fourth, fifth, and
sixth impingers are not used.
6.1.6 Metering
System, Barometer, and Gas Density Determination Equipment. Same as Method 5,
Sections 6.1.1.9, 6.1.2, and 6.1.3, respectively.
6.1.7 Teflon Tape.
For capping openings and sealing connections, if necessary, on the sampling
train.
6.2 Sample Recovery.
Same as Method 5, Sections 6.2.1 through 6.2.8
(Probe-Liner and Probe-Nozzle Brushes or Swabs, Wash Bottles, Sample Storage
Containers, Petri Dishes, Glass Graduated Cylinder, Plastic Storage Containers,
Funnel and Rubber Policeman, and Glass Funnel), respectively, with the
following exceptions and additions:
6.2.1 Non-metallic
Probe-Liner and Probe-Nozzle Brushes or Swabs. Use non-metallic probe-liner and
probe nozzle brushes or swabs for quantitative recovery of materials collected
in the front-half of the sampling train.
6.2.2 Sample Storage
Containers. Use glass bottles (see Section 8.1 of this Method) with Teflon-lined
caps that are non-reactive to the oxidizing solutions, with capacities of 1000-
and 500-ml, for storage of acidified KMnO4-
containing samples and blanks. Glass or polyethylene bottles may be used for
other sample types.
6.2.3 Graduated Cylinder.
Glass or equivalent.
6.2.4 Funnel. Glass
or equivalent.
6.2.5 Labels. For
identifying samples.
6.2.6 Polypropylene
Tweezers and/or Plastic Gloves. For recovery of the filter from the sampling
train filter holder.
6.3 Sample Preparation and Analysis.
6.3.1 Volumetric
Flasks, 100-ml, 250-ml, and 1000-ml. For preparation of standards and sample
dilutions.
6.3.2 Graduated
Cylinders. For preparation of reagents.
6.3.3 Parr Bombs or
Microwave Pressure Relief Vessels with Capping Station (CEM Corporation model
or equivalent). For sample digestion.
6.3.4 Beakers and
Watch Glasses. 250-ml beakers, with watch glass covers, for sample digestion.
6.3.5 Ring Stands and
Clamps. For securing equipment such as filtration apparatus.
6.3.6 Filter Funnels.
For holding filter paper.
6.3.7 Disposable
Pasteur Pipets and Bulbs.
6.3.8 Volumetric
Pipets.
6.3.9 Analytical
Balance. Accurate to within 0.1 mg.
6.3.10 Microwave or
Conventional Oven. For heating samples at fixed power levels or temperatures,
respectively.
6.3.11 Hot Plates.
6.3.12 Atomic
Absorption Spectrometer (AAS). Equipped with a background corrector.
6.3.12.1 Graphite
Furnace Attachment. With Sb, As, Cd, Co, Pb, Se, and Tl hollow cathode lamps
(HCLs) or electrodeless discharge lamps (EDLs). Same as Reference 2 in Section
16.0. Methods 7041 (Sb), 7060 (As), 7131 (Cd), 7201 (Co), 7421 (Pb), 7740 (Se),
and 7841 (Tl).
6.3.12.2 Cold Vapor
Mercury Attachment. With a mercury HCL or EDL, an air recirculation pump, a
quartz cell, an aerator apparatus, and a heat lamp or desiccator tube. The heat
lamp shall be capable of raising the temperature at the quartz cell by 1OûC
above ambient, so that no condensation forms on the wall of the quartz cell.
Same as Method 7470 in Reference 2 in Section 16.0. See NOTE 2: Section 11.1.3 for other acceptable approaches
for analysis of Hg in which analytical detection limits of 0.002 ng/ml were
obtained.
6.3.13 Inductively
Coupled Argon Plasma Spectrometer. With either a direct or sequential reader
and an alumina torch. Same as EPA Method 6010 in Reference 2 in Section 16.0.
6.3.14 Inductively
Coupled Plasma-Mass Spectrometer. Same as EPA Method 6020 in Reference 2 in
Section 16.0.
7.0 Reagents and Standards.
7.1 Conform to the Specifications
Unless otherwise
indicated, it is intended that all reagents conform to the specifications
established by the Committee on Analytical Reagents of the American Chemical
Society, where such specifications are available. Otherwise, use the best
available grade.
7.2 Sampling Reagents.
7.2.1 Sample Filters.
Without organic binders. The filters shall contain less than 1.3 µg/in.2 of each of the metals to be measured. Analytical results provided
by filter manufacturers stating metals content of the filters are acceptable.
However, if no such results are available, analyze filter blanks for each
target metal prior to emission testing. Quartz fiber filters meeting these
requirements are recommended. However, if glass fiber filters become available
which meet these requirements, they may be used. Filter efficiencies and
unreactiveness to sulfur dioxide (SO2) or
sulfur trioxide (SO3) shall be as described in Section 7.1.1 of Method 5.
7.2.2 Water. To
conform to ASTM Specification D1193-77 or 91, Type II (incorporated by
reference -- see ¤60.17). If necessary, analyze the water for all target metals
prior to field use. All target metals should be less than 1 ng/ml.
7.2.3 HNO3, Concentrated. Baker Instra-analyzed or equivalent.
7.2.4 HCl,
Concentrated. Baker Instra-analyzed or equivalent.
7.2.5 H2O2, 30 Percent (V/V).
7.2.6 KMnO4.
7.2.7 H2SO4, Concentrated.
7.2.8 Silica Gel and Crushed
Ice. Same as Method 5, Sections 7.1.2 and 7.1.4, respectively.
7.3 Pretest Preparation of Sampling Reagents.
7.3.1 HNO3/H2O2 Absorbing Solution, 5
Percent HNO3/10 Percent H2O2. Add carefully with stirring 50 ml of concentrated HNO3 to a 1000-ml volumetric flask containing approximately 500 ml of
water, and then add carefully with stirring 333 ml of 30 percent H2O2. Dilute to volume with water. Mix well. This
reagent shall contain less than 2 ng/ml of each target metal.
7.3.2
Acidic KMnO4 Absorbing Solution, 4 Percent KMnO4 (W/V), 10 Percent H2SO4 (V/V). Prepare fresh daily. Mix carefully, with stirring, 100 ml of
concentrated H2SO4 into
approximately 800 ml of water, and add water with stirring to make a volume of
1 liter: this solution is 10 percent H2SO4 (V/V). Dissolve, with stirring, 40 g of KMnO4 into 10 percent H2SO4 (V/V) and add 10 percent H2SO4 (V/V) with stirring to make a volume of 1 liter. Prepare and store
in glass bottles to prevent degradation. This reagent shall contain less than 2
ng/ml of Hg.
Precaution: To prevent autocatalytic decomposition of the
permanganate solution, filter the solution through Whatman 541 filter paper.
7.3.3 HNO3, 0.1 N. Add with stirring 6.3 ml of concentrated HNO3 (70 percent) to a flask containing approximately 900 ml of water.
Dilute to 1000 ml with water. Mix well. This reagent shall contain less than 2
ng/ml of each target metal.
7.3.4 HCl, 8 N.
Carefully add with stirring 690 ml of concentrated HCl to a flask containing
250 ml of water. Dilute to 1000 ml with water. Mix well. This reagent shall
contain less than 2 ng/ml of Hg.
7.4 Glassware Cleaning Reagents.
7.4.1 HNO3, Concentrated. Fisher ACS grade or equivalent.
7.4.2 Water. To
conform to ASTM Specifications D1193, Type II.
7.4.3 HNO3, 10 Percent (V/V). Add with stirring 500 ml of concentrated HNO3 to a flask containing approximately 4000 ml of water. Dilute to
5000 ml with water. Mix well. This reagent shall contain less than 2 ng/ml of
each target metal.
7.5 Sample Digestion and Analysis Reagents.
The metals standards,
except Hg, may also be made from solid chemicals as described in Reference 3 in
Section 16.0. Refer to References 1, 2, or 5 in Section 16.0 for additional
information on Hg standards. The 1000 µg/ml Hg stock solution standard may be
made according to Section 7.2.7 of Method 101A.
7.5.1 HCl,
Concentrated.
7.5.2 HF,
Concentrated.
7.5.3 HNO3, Concentrated. Baker Instra-analyzed or equivalent.
7.5.4 HNO3, 50 Percent (V/V). Add with stirring 125 ml of concentrated HNO3 to 100 ml of water. Dilute to 250 ml with water. Mix well. This
reagent shall contain less than 2 ng/ml of each target metal.
7.5.5 HNO3, 5 Percent (V/V). Add with stirring 50 ml of concentrated HNO3 to 800 ml of water. Dilute to 1000 ml with water. Mix well. This
reagent shall contain less than 2 ng/ml of each target metal.
7.5.6 Water. To
conform to ASTM Specifications D1193, Type II.
7.5.7 Hydroxylamine
Hydrochloride and Sodium Chloride Solution. See Reference 2 In Section 16.0 for preparation.
7.5.8 Stannous
Chloride. See Reference 2 in Section 16.0 for preparation.
7.5.9 KMnO4, 5 Percent (W/V). See Reference 2 in Section 16.0 for preparation.
7.5.10 H2SO4, Concentrated.
7.5.11 Potassium
Persulfate, 5 Percent (W/V). See Reference 2 in Section 16.0 for preparation.
7.5.12 Nickel
Nitrate, Ni(N03)2 .6H20.
7.5.13 Lanthanum
Oxide, La203.
7.5.14 Hg Standard
(AAS Grade), 1000 µg/ml.
7.5.15 Pb Standard (AAS
Grade), 1000 µg/ml.
7.5.16 As Standard
(AAS Grade), 1000 µg/ml.
7.5.17 Cd Standard
(AAS Grade), 1000 µg/ml.
7.5.18 Cr Standard
(AAS Grade), 1000 µg/ml.
7.5.19 Sb Standard
(AAS Grade), 1000 µg/ml.
7.5.20 Ba Standard
(AAS Grade), 1000 µg/ml.
7.5.21 Be Standard
(AAS Grade), 1000 µg/ml.
7.5.22 Co Standard
(AAS Grade), 1000 µg/ml.
7.5.23 Cu Standard
(AAS Grade), 1000 µg/ml.
7.5.24 Mn Standard
(AAS Grade), 1000 µg/ml.
7.5.25 Ni Standard
(AAS Grade), 1000 µg/ml.
7.5.26 P Standard (AAS
Grade), 1000 µg/ml.
7.5.27 Se Standard
(AAS Grade), 1000 µg/ml.
7.5.28 Ag Standard
(AAS Grade), 1000 µg/ml.
7.5.29 Tl Standard
(AAS Grade), 1000 µg/ml.
7.5.30 Zn Standard
(AAS Grade), 1000 µg/ml.
7.5.31 Al Standard
(AAS Grade), 1000 µg/ml.
7.5.32 Fe Standard
(AAS Grade), 1000 µg/ml.
7.5.33 Hg Standards
and Quality Control Samples. Prepare fresh weekly a 10 µg/ml intermediate Hg
standard by adding 5 ml of 1000 µg/ml Hg stock solution prepared according to
Method 101A to a 500-ml volumetric flask; dilute with stirring to 500 ml by
first carefully adding 20 ml of 15 percent HNO3 and
then adding water to the 500-ml volume. Mix well. Prepare a 200 ng/ml working
Hg standard solution fresh daily: add 5 ml of the 10 µg/ml intermediate
standard to a 250-ml volumetric flask, and dilute to 250 ml with 5 ml of 4
percent KMnO4, 5 ml of 15 percent HNO3, and then water. Mix well. Use at least five separate aliquots of
the working Hg standard solution and a blank to prepare the standard curve.
These aliquots and blank shall contain 0.0, 1.0, 2.0, 3.0, 4.0, and 5.0 ml of
the working standard solution containing 0, 200, 400, 600, 800, and 1000 ng Hg,
respectively. Prepare quality control samples by making a separate 10 µg/ml
standard and diluting until in the calibration range.
7.5.34 ICAP Standards
and Quality Control Samples. Calibration standards for ICAP analysis can be
combined into four different mixed standard solutions as follows:
MIXED
STANDARD SOLUTIONS FOR ICAP ANALYSIS
Solution
Elements
I As,
Be, Cd, Mn,
Pb, Se, Zn
II Ba,
Co, Cu, Fe
III Al,
Cr, Ni
IV Ag,
P, Sb, Tl
Prepare these
standards by combining and diluting the appropriate volumes of the 1000 µg/ml
solutions with 5 percent HNO3. A minimum of one standard
and a blank can be used to form each calibration curve. However, prepare a
separate quality control sample spiked with known amounts of the target metals
in quantities in the mid-range of the calibration curve. Suggested standard
levels are 25 µg/ml for Al, Cr and Pb, 15 µg/ml for Fe, and 10 µg/ml for the
remaining elements. Prepare any standards containing less than 1 µg/ml of metal
on a daily basis. Standards containing greater than 1 µg/ml of metal should be
stable for a minimum of 1 to 2 weeks. For ICP-MS, follow Method 6020 in EPA
Publication SW-846 Third Edition (November 1986) including updates I, II, IIA,
IIB and III, as incorporated by reference in ¤60.17(i).
7.5.35 GFAAS
Standards. Sb, As, Cd, Co, Pb, Se, and Tl. Prepare a 10 µg/ml standard by
adding 1 ml of 1000 µg/ml standard to a 100-ml volumetric flask. Dilute with
stirring to 100 ml with 10 percent HNO3. For
GFAAS, matrix match the standards. Prepare a 100 ng/ml standard by adding 1 ml
of the 10 µg/ml standard to a 100-ml volumetric flask, and dilute to 100 ml
with the appropriate matrix solution. Prepare other standards by diluting the
100 ng/ml standards. Use at least five standards to make up the standard curve.
Suggested levels are 0, 10, 50, 75, and 100 ng/ml. Prepare quality control
samples by making a separate 10 µg/ml standard and diluting until it is in the
range of the samples. Prepare any standards containing less than 1 µg/ml of
metal on a daily basis. Standards containing greater than 1 µg/ml of metal
should be stable for a minimum of 1 to 2 weeks.
7.5.36 Matrix
Modifiers.
7.5.36.1 Nickel
Nitrate, 1 Percent (V/V). Dissolve 4.956 g of Ni(N03)2á6H20 or other
nickel compound suitable for preparation of this matrix modifier in
approximately 50 ml of water in a 100-ml volumetric flask. Dilute to 100 ml
with water.
7.5.36.2 Nickel
Nitrate, 0.1 Percent (V/V). Dilute 10 ml of 1 percent nickel nitrate solution
to 100 ml with water. Inject an equal amount of sample and this modifier into
the graphite furnace during GFAAS analysis for As.
7.5.36.3 Lanthanum.
Carefully dissolve 0.5864 g of La203 in 10 ml of concentrated HN03, and
dilute the solution by adding it with stirring to approximately 50 ml of water.
Dilute to 100 ml with water, and mix well. Inject an equal amount of sample and
this modifier into the graphite furnace during GFAAS analysis for Pb.
7.5.37 Whatman 40 and
541 Filter Papers (or equivalent). For filtration of digested samples.
8.0 Sample Collection, Preservation, Transport, and Storage.
8.1 Sampling.
The complexity of
this method is such that, to obtain reliable results, both testers and analysts
must be trained and experienced with the test procedures, including source
sampling; reagent preparation and handling; sample handling; safety equipment
and procedures; analytical calculations; reporting; and the specific procedural
descriptions throughout this method.
8.1.1 Pretest
Preparation. Follow the same general procedure given in Method 5, Section 8.1,
except that, unless particulate emissions are to be determined, the filter need
not be desiccated or weighed. First, rinse all sampling train glassware with
hot tap water and then wash in hot soapy water. Next, rinse glassware three
times with tap water, followed by three additional rinses with water. Then soak
all glassware in a 10 percent (V/V) nitric acid solution for a minimum of 4
hours, rinse three times with water, rinse a final time with acetone, and allow
to air dry. Cover all glassware openings where contamination can occur until
the sampling train is assembled for sampling.
8.1.2 Preliminary
Determinations. Same as Method 5, Section 8.1.2.
8.1.3 Preparation of
Sampling Train.
8.1.3.1 Set up the
sampling train as shown in Figure 29-1. Follow the same general procedures
given in Method 5, Section 8.3, except place
100 ml of the HNO3/H2O2 solution (Section 7.3.1 of this method) in each of the second and
third impingers as shown in Figure 29-1. Place 100 ml of the acidic KMnO4 absorbing solution (Section 7.3.2 of this method) in each of the
fifth and sixth impingers as shown in Figure 29-1, and transfer approximately
200 to 300 g of preweighed silica gel from its container to the last impinger.
Alternatively, the silica gel may be weighed directly in the impinger just
prior to final train assembly.
8.1.3.2 Based on the
specific source sampling conditions, the use of an empty first impinger can be
eliminated if the moisture to be collected in the impingers will be less than
approximately 100 ml.
8.1.3.3 If Hg
analysis will not be performed, the fourth, fifth, and sixth impingers as shown
in Figure 29-1 are not required.
8.1.3.4 To insure
leak-free sampling train connections and to prevent possible sample
contamination problems, use Teflon tape or other non-contaminating material
instead of silicone grease.
Precaution: Exercise extreme care to prevent contamination
within the train. Prevent the acidic KMnO4 from
contacting any glassware that contains sample material to be analyzed for Mn.
Prevent acidic H2O2 from mixing with the
acidic KMnO4.
8.1.4 Leak-Check
Procedures. Follow the leak-check procedures given in Method 5, Section 8.4.2 (Pretest Leak-Check),
Section 8.4.3 (Leak-Checks During the Sample Run), and Section 8.4.4 (Post-Test
Leak-Checks).
8.1.5 Sampling Train
Operation. Follow the procedures given in Method
5, Section 8.5. When sampling for Hg, use a procedure analogous to that
described in Section 8.1 of Method 101A, 40 CFR Part 61, Appendix B, if
necessary to maintain the desired color in the last acidified permanganate
impinger. For each run, record the data required on a data sheet such as the
one shown in Figure 5-3 of Method 5.
8.1.6 Calculation of
Percent Isokinetic. Same as Method 5, Section
12.11.
8.2 Sample Recovery.
8.2.1 Begin cleanup
procedures as soon as the probe is removed from the stack at the end of a
sampling period. The probe should be allowed to cool prior to sample recovery.
When it can be safely handled, wipe off all external particulate matter near
the tip of the probe nozzle and place a rinsed, non-contaminating cap over the
probe nozzle to prevent losing or gaining particulate matter. Do not cap the
probe tip tightly while the sampling train is cooling; a vacuum can form in the
filter holder with the undesired result of drawing liquid from the impingers
onto the filter.
8.2.2 Before moving
the sampling train to the cleanup site, remove the probe from the sampling
train and cap the open outlet. Be careful not to lose any condensate that might
be present. Cap the filter inlet where the probe was fastened. Remove the umbilical
cord from the last impinger and cap the impinger. Cap the filter holder outlet
and impinger inlet. Use non-contaminating caps, whether ground glass stoppers,
plastic caps, serum caps, or Teflon¨ tape to close these openings.
8.2.3 Alternatively,
the following procedure may be used to disassemble the train before the probe
and filter holder/oven are completely cooled: Initially disconnect the filter
holder outlet/impinger inlet and loosely cap the open ends. Then disconnect the
probe from the filter holder or cyclone inlet and loosely cap the open ends.
Cap the probe tip and remove the umbilical cord as previously described.
8.2.4 Transfer the
probe and filter-impinger assembly to a cleanup area that is clean and protected
from the wind and other potential causes of contamination or loss of sample.
Inspect the train before and during disassembly and note any abnormal
conditions. Take special precautions to assure that all the items necessary for
recovery do not contaminate the samples. The sample is recovered and treated as
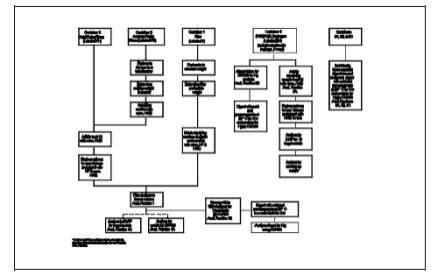
follows (see schematic in Figures 29-2a and 29-2b):
8.2.5 Container No. 1
(Sample Filter). Carefully remove the filter from the filter holder and place
it in its labeled Petri dish container. To handle the filter, use either
acid-washed polypropylene or Teflon coated tweezers or clean, disposable
surgical gloves rinsed with water and dried. If it is necessary to fold the
filter, make certain the particulate cake is inside the fold. Carefully
transfer the filter and any particulate matter or filter fibers that adhere to
the filter holder gasket to the Petri dish by using a dry (acid-cleaned) nylon
bristle brush. Do not use any metal-containing materials when recovering this
train. Seal the labeled Petri dish.
8.2.6 Container No. 2
(Acetone Rinse). Perform this procedure only if a determination of particulate
emissions is to be made. Quantitatively recover particulate matter and any
condensate from the probe nozzle, probe fitting, probe liner, and front half of
the filter holder by washing these components with a total of 100 ml of
acetone, while simultaneously taking great care to see that no dust on the
outside of the probe or other surfaces gets in the sample. The use of exactly
100 ml is necessary for the subsequent blank correction procedures. Distilled
water may be used instead of acetone when approved by the Administrator and
shall be used when specified by the Administrator; in these cases, save a water
blank and follow the Administrator's directions on analysis.
8.2.6.1 Carefully
remove the probe nozzle, and clean the inside surface by rinsing with acetone
from a wash bottle while brushing with a non-metallic brush. Brush until the
acetone rinse shows no visible particles, then make a final rinse of the inside
surface with acetone.
8.2.6.2 Brush and
rinse the sample exposed inside parts of the probe fitting with acetone in a
similar way until no visible particles remain. Rinse the probe liner with
acetone by tilting and rotating the probe while squirting acetone into its
upper end so that all inside surfaces will be wetted with acetone. Allow the
acetone to drain from the lower end into the sample container. A funnel may be
used to aid in transferring liquid washings to the container. Follow the
acetone rinse with a non-metallic probe brush. Hold the probe in an inclined
position, squirt acetone into the upper end as the probe brush is being pushed
with a twisting action three times through the probe. Hold a sample container
underneath the lower end of the probe, and catch any acetone and particulate
matter which is brushed through the probe until no visible particulate matter
is carried out with the acetone or until none remains in the probe liner on
visual inspection. Rinse the brush with acetone, and quantitatively collect
these washings in the sample container. After the brushing, make a final
acetone rinse of the probe as described above.
8.2.6.3 It is
recommended that two people clean the probe to minimize sample losses. Between
sampling runs, keep brushes clean and protected from contamination. Clean the
inside of the front-half of the filter holder by rubbing the surfaces with a
non-metallic brush and rinsing with acetone. Rinse each surface three times or
more if needed to remove visible particulate. Make a final rinse of the brush
and filter holder. After all acetone washings and particulate matter have been
collected in the sample container, tighten the lid so that acetone will not
leak out when shipped to the laboratory. Mark the height of the fluid level to
determine whether or not leakage occurred during transport. Clearly label the
container to identify its contents.
8.2.7 Container No. 3
(Probe Rinse). Keep the probe assembly clean and free from contamination during
the probe rinse. Rinse the probe nozzle and fitting, probe liner, and
front-half of the filter holder thoroughly with a total of 100 ml of 0.1 N HNO3, and place the wash into a sample storage container. Perform the
rinses as applicable and generally as described in Method 12, Section 8.7.1.
Record the volume of the rinses. Mark the height of the fluid level on the
outside of the storage container and use this mark to determine if leakage
occurs during transport. Seal the container, and clearly label the contents.
Finally, rinse the nozzle, probe liner, and front-half of the filter holder
with water followed by acetone, and discard these rinses.
NOTE: The use of a total of exactly 100 ml is
necessary for the subsequent blank correction procedures.
8.2.8 Container No. 4
(Impingers 1 through 3, Moisture Knockout Impinger, when used, HNO3/H2O2 Impingers Contents
and Rinses). Due to the potentially large quantity of liquid involved, the
tester may place the impinger solutions from impingers 1 through 3 in more than
one container, if necessary. Measure the liquid in the first three impingers to
within 0.5 ml using a graduated cylinder. Record the volume. This information
is required to calculate the moisture content of the sampled flue gas. Clean
each of the first three impingers, the filter support, the back half of the
filter housing, and connecting glassware by thoroughly rinsing with 100 ml of
0.1 N HNO3 using the procedure as applicable in Method 12,
Section 8.7.3.
NOTE: The use of exactly 100 ml of 0.1 N HNO3 rinse is necessary for the subsequent blank correction procedures.
Combine the rinses and impinger solutions, measure and record the final total
volume. Mark the height of the fluid level, seal the container, and clearly
label the contents.
8.2.9 Container Nos.
5A (0.1 N HNO3), 5B (KMnO4/H2SO4 absorbing solution), and 5C (8 N HCl rinse and
dilution).
8.2.9.1 When sampling
for Hg, pour all the liquid from the impinger (normally impinger No. 4) that
immediately preceded the two permanganate impingers into a graduated cylinder
and measure the volume to within 0.5 ml. This information is required to
calculate the moisture content of the sampled flue gas. Place the liquid in
Container No. 5A. Rinse the impinger with exactly 100 ml of 0.1 N HNO3 and place this rinse in Container No. 5A.
8.2.9.2 Pour all the
liquid from the two permanganate impingers into a graduated cylinder and
measure the volume to within 0.5 ml. This information is required to calculate
the moisture content of the sampled flue gas. Place this acidic KMnO4 solution into Container No. 5B. Using a total of exactly 100 ml of
fresh acidified KMnO4
solution for all rinses
(approximately 33 ml per rinse), rinse the two permanganate impingers and
connecting glassware a minimum of three times. Pour the rinses into Container
No. 5B, carefully assuring transfer of all loose precipitated materials from
the two impingers. Similarly, using 100 ml total of water, rinse the
permanganate impingers and connecting glass a minimum of three times, and pour
the rinses into Container 5B, carefully assuring transfer of any loose
precipitated material. Mark the height of the fluid level, and clearly label
the contents. Read the Precaution: in
Section 7.3.2.
NOTE: Due to the potential reaction of KMnO4 with acid, pressure buildup can occur in the sample storage
bottles. Do not fill these bottles completely and take precautions to relieve
excess pressure. A No. 70-72 hole drilled in the container cap and Teflon liner
has been used successfully.
8.2.9.3 If no visible
deposits remain after the water rinse, no further rinse is necessary. However,
if deposits remain on the impinger surfaces, wash them with 25 ml of 8 N HCl, and
place the wash in a separate sample container labeled No. 5C containing 200 ml
of water. First, place 200 ml of water in the container. Then wash the impinger
walls and stem with the HCl by turning the impinger on its side and rotating it
so that the HCl contacts all inside surfaces. Use a total of only 25 ml of 8 N
HCl for rinsing both permanganate impingers combined. Rinse the first impinger,
then pour the actual rinse used for the first impinger into the second impinger
for its rinse. Finally, pour the 25 ml of 8 N HCl rinse carefully into the
container. Mark the height of the fluid level on the outside of the container
to determine if leakage occurs during transport.
8.2.10 Container No.
6 (Silica Gel). Note the color of the indicating silica gel to determine
whether it has been completely spent and make a notation of its condition.
Transfer the silica gel from its impinger to its original container and seal
it. The tester may use a funnel to pour the silica gel and a rubber policeman
to remove the silica gel from the impinger. The small amount of particles that
might adhere to the impinger wall need not be removed. Do not use water or
other liquids to transfer the silica gel since weight gained in the silica gel
impinger is used for moisture calculations. Alternatively, if a balance is
available in the field, record the weight of the spent silica gel (or silica
gel plus impinger) to the nearest 0.5 g.
8.2.11 Container No.
7 (Acetone Blank). If particulate emissions are to be determined, at least once
during each field test, place a 100-ml portion of the acetone used in the
sample recovery process into a container labeled No. 7. Seal the container.
8.2.12 Container No.
8A (0.1 N HNO3 Blank). At least once during each field test,
place 300 ml of the 0.1 N HNO3 solution used in the
sample recovery process into a container labeled No. 8A. Seal the container.
8.2.13 Container No.
8B (Water Blank). At least once during each field test, place 100 ml of the
water used in the sample recovery process into a container labeled No. 8B. Seal
the container.
8.2.14 Container No.
9 (5 Percent HNO3/10 Percent H2O2 Blank). At least once during each field test, place 200 ml of the 5
Percent HNO3/10 Percent H2O2 solution used as the nitric acid impinger reagent into a container
labeled No. 9. Seal the container.
8.2.15 Container No.
10 (Acidified KMnO4
Blank). At least once during each
field test, place 100 ml of the acidified KMnO4 solution
used as the impinger solution and in the sample recovery process into a
container labeled No. 10. Prepare the container as described in Section
8.2.9.2. Read the Precaution: in
Section 7.3.2 and read the NOTE in Section 8.2.9.2.
8.2.16 Container No.
11 (8 N HCl Blank). At least once during each field test, place 200 ml of water
into a sample container labeled No. 11. Then carefully add with stirring 25 ml
of 8 N HCl. Mix well and seal the container.
8.2.17 Container No.
12 (Sample Filter Blank). Once during each field test, place into a Petri dish
labeled No. 12 three unused blank filters from the same lot as the sampling
filters. Seal the Petri dish.
8.3 Sample Preparation.
Note the level of the
liquid in each of the containers and determine if any sample was lost during
shipment. If a noticeable amount of leakage has occurred, either void the
sample or use methods, subject to the approval of the Administrator, to correct
the final results. A diagram illustrating sample preparation and analysis
procedures for each of the sample train components is shown in Figure 29-3.
8.3.1 Container No. 1
(Sample Filter).
8.3.1.1 If
particulate emissions are being determined, first desiccate the filter and
filter catch without added heat (do not heat the filters to speed the drying)
and weigh to a constant weight as described in Section
11.2.1 of Method 5.
8.3.1.2 Following
this procedure, or initially, if particulate emissions are not being determined
in addition to metals analysis, divide the filter with its filter catch into
portions containing approximately 0.5 g each. Place the pieces in the analyst's
choice of either individual microwave pressure relief vessels or Parr Bombs.
Add 6 ml of concentrated HNO3 and 4 ml of
concentrated HF to each vessel. For microwave heating, microwave the samples
for approximately 12 to 15 minutes total heating time as follows: heat for 2 to
3 minutes, then turn off the microwave for 2 to 3 minutes, then heat for 2 to 3
minutes, etc., continue this alternation until the 12 to 15 minutes total
heating time are completed (this procedure should comprise approximately 24 to
30 minutes at 600 watts). Microwave heating times are approximate and are
dependent upon the number of samples being digested simultaneously. Sufficient
heating is evidenced by sorbent reflux within the vessel. For conventional
heating, heat the Parr Bombs at 140oC (285oF) for 6
hours. Then cool the samples to room temperature, and combine with the acid
digested probe rinse as required in Section 8.3.3.
8.3.1.3 If the
sampling train includes an optional glass cyclone in front of the filter, prepare
and digest the cyclone catch by the procedures described in Section 8.3.1.2 and
then combine the digestate with the digested filter sample.
8.3.2 Container No. 2
(Acetone Rinse). Note the level of liquid in the container and confirm on the analysis
sheet whether or not leakage occurred during transport. If a noticeable amount
of leakage has occurred, either void the sample or use methods, subject to the
approval of the Administrator, to correct the final results. Measure the liquid
in this container either volumetrically within 1 ml or gravimetrically within
0.5 g. Transfer the contents to an acid-cleaned, tared 250-ml beaker and
evaporate to dryness at ambient temperature and pressure. If particulate
emissions are being determined, desiccate for 24 hours without added heat,
weigh to a constant weight according to the procedures described in Section
11.2.1 of Method 5, and report the results to the nearest 0.1 mg. Redissolve
the residue with 10 ml of concentrated HNO3.
Quantitatively combine the resultant sample, including all liquid and any
particulate matter, with Container No. 3 before beginning Section 8.3.3.
8.3.3 Container No. 3
(Probe Rinse). Verify that the pH of this sample is 2 or lower. If it is not,
acidify the sample by careful addition with stirring of concentrated HNO3 to pH 2. Use water to rinse the sample into a beaker, and cover the
beaker with a ribbed watch glass. Reduce the sample volume to approximately 20
ml by heating on a hot plate at a temperature just below boiling. Digest the
sample in microwave vessels or Parr Bombs by quantitatively transferring the
sample to the vessel or bomb, carefully adding the 6 ml of concentrated HNO3, 4 ml of concentrated HF, and then continuing to follow the
procedures described in Section 8.3.1.2. Then combine the resultant sample
directly with the acid digested portions of the filter prepared previously in
Section 8.3.1.2. The resultant combined sample is referred to as "Sample
Fraction 1". Filter the combined sample using Whatman 541 filter paper.
Dilute to 300 ml (or the appropriate volume for the expected metals
concentration) with water. This diluted sample is "Analytical Fraction
1". Measure and record the volume of Analytical Fraction 1 to within 0.1
ml. Quantitatively remove a 50-ml aliquot and label as "Analytical
Fraction 1B". Label the remaining 250-ml portion as "Analytical
Fraction 1A". Analytical Fraction 1A is used for ICAP or AAS analysis for
all desired metals except Hg. Analytical Fraction 1B is used for the
determination of front-half Hg.
8.3.4 Container No. 4
(Impingers 1-3). Measure and record the total volume of this sample to within
0.5 ml and label it "Sample Fraction 2". Remove a 75- to 100-ml
aliquot for Hg analysis and label the aliquot "Analytical Fraction
2B". Label the remaining portion of Container No. 4 as "Sample
Fraction 2A". Sample Fraction 2A defines the volume of Analytical Fraction
2A prior to digestion. All of Sample Fraction 2A is digested to produce
"Analytical Fraction 2A". Analytical Fraction 2A defines the volume
of Sample Fraction 2A after its digestion and the volume of Analytical Fraction
2A is normally 150 ml. Analytical Fraction 2A is analyzed for all metals except
Hg. Verify that the pH of Sample Fraction 2A is 2 or lower. If necessary, use
concentrated HNO3 by careful addition and stirring to lower Sample
Fraction 2A to pH 2. Use water to rinse Sample Fraction 2A into a beaker and
then cover the beaker with a ribbed watch glass. Reduce Sample Fraction 2A to
approximately 20 ml by heating on a hot plate at a temperature just below
boiling. Then follow either of the digestion procedures described in Sections
8.3.4.1 or 8.3.4.2.
8.3.4.1 Conventional
Digestion Procedure. Add 30 ml of 50 percent HNO3,
and heat for 30 minutes on a hot plate to just below boiling. Add 10 ml of 3
percent H2O2 and heat for 10 more
minutes. Add 50 ml of hot water, and heat the sample for an additional 20
minutes. Cool, filter the sample, and dilute to 150 ml (or the appropriate
volume for the expected metals concentrations) with water. This dilution
produces Analytical Fraction 2A. Measure and record the volume to within 0.1
ml.
8.3.4.2 Microwave
Digestion Procedure. Add 10 ml of 50 percent HNO3 and
heat for 6 minutes total heating time in alternations of 1 to 2 minutes at 600
Watts followed by 1 to 2 minutes with no power, etc., similar to the procedure
described in Section 8.3.1. Allow the sample to cool. Add 10 ml of 3 percent H2O2 and heat for 2 more minutes. Add 50 ml of hot
water, and heat for an additional 5 minutes. Cool, filter the sample, and
dilute to 150 ml (or the appropriate volume for the expected metals
concentrations) with water. This dilution produces Analytical Fraction 2A.
Measure and record the volume to within 0.1 ml.
NOTE: All microwave heating times given are
approximate and are dependent upon the number of samples being digested at a
time. Heating times as given above have been found acceptable for simultaneous
digestion of up to 12 individual samples. Sufficient heating is evidenced by
solvent reflux within the vessel.
8.3.5 Container No.
5A (Impinger 4), Container Nos. 5B and 5C (Impingers 5 and 6). Keep the samples
in Containers Nos. 5A, 5B, and 5C separate from each other. Measure and record
the volume of 5A to within 0.5 ml. Label the contents of Container No. 5A to be
Analytical Fraction 3A. To remove any brown MnO2 precipitate
from the contents of Container No. 5B, filter its contents through Whatman 40
filter paper into a 500 ml volumetric flask and dilute to volume with water.
Save the filter for digestion of the brown MnO2 precipitate.
Label the 500 ml filtrate from Container No. 5B to be Analytical Fraction 3B.
Analyze Analytical Fraction 3B for Hg within 48 hours of the filtration step.
Place the saved filter, which was used to remove the brown MnO2 precipitate, into an appropriately sized vented container, which
will allow release of any gases including chlorine formed when the filter is
digested. In a laboratory hood which will remove any gas produced by the
digestion of the MnO2, add 25 ml of 8 N HCl to the filter and allow
to digest for a minimum of 24 hours at room temperature. Filter the contents of
Container No. 5C through a Whatman 40 filter into a 500-ml volumetric flask.
Then filter the result of the digestion of the brown MnO2 from Container No. 5B through a Whatman 40 filter into the same
500-ml volumetric flask, and dilute and mix well to volume with water. Discard
the Whatman 40 filter. Mark this combined 500-ml dilute HCl solution as
Analytical Fraction 3C.
8.3.6 Container No. 6
(Silica Gel). Weigh the spent silica gel (or silica gel plus impinger) to the
nearest 0.5 g using a balance.
9.0 Quality Control.
9.1 Field Reagent Blanks, if analyzed.
Perform the digestion
and analysis of the blanks in Container Nos. 7 through 12 that were produced in
Sections 8.2.11 through 8.2.17, respectively. For Hg field reagent blanks, use
a 10 ml aliquot for digestion and analysis.
9.1.1 Digest and
analyze one of the filters from Container No. 12 per Section 8.3.1, 100 ml from
Container No. 7 per Section 8.3.2, and 100 ml from Container No. 8A per Section
8.3.3. This step produces blanks for Analytical Fractions 1A and 1B.
9.1.2 Combine 100 ml
of Container No. 8A with 200 ml from Container No. 9, and digest and analyze
the resultant volume per Section 8.3.4. This step produces blanks for
Analytical Fractions 2A and 2B.
9.1.3 Digest and
analyze a 100-ml portion of Container No. 8A to produce a blank for Analytical
Fraction 3A. 9.1.4 Combine 100 ml from Container No. 10 with 33 ml from
Container No. 8B to produce a blank for Analytical Fraction 3B. Filter the
resultant 133 ml as described for Container No. 5B in Section 8.3.5, except do
not dilute the 133 ml. Analyze this blank for Hg within 48 hr of the filtration
step, and use 400 ml as the blank volume when calculating the blank mass value.
Use the actual volumes of the other analytical blanks when calculating their
mass values.
9.1.5 Digest the
filter that was used to remove any brown MnO2 precipitate
from the blank for Analytical Fraction 3B by the same procedure as described in
Section 8.3.5 for the similar sample filter. Filter the digestate and the
contents of Container No. 11 through Whatman 40 paper into a 500-ml volumetric
flask, and dilute to volume with water. These steps produce a blank for
Analytical Fraction 3C.
9.1.6 Analyze the
blanks for Analytical Fraction Blanks 1A and 2A per Section 11.1.1 and/or
Section 11.1.2. Analyze the blanks for Analytical Fractions 1B, 2B, 3A, 3B, and
3C per Section 11.1.3. Analysis of the blank for Analytical Fraction 1A
produces the front-half reagent blank correction values for the desired metals
except for Hg; Analysis of the blank for Analytical Fraction 1B produces the
front-half reagent blank correction value for Hg. Analysis of the blank for
Analytical Fraction 2A produces the back-half reagent blank correction values
for all of the desired metals except for Hg, while separate analyses of the
blanks for Analytical Fractions 2B, 3A, 3B, and 3C produce the back-half
reagent blank correction value for Hg.
9.2 Quality Control Samples.
Analyze the following
quality control samples.
9.2.1 ICAP and ICP-MS
Analysis. Follow the respective quality control descriptions in Section 8 of
Methods 6010 and 6020 in EPA Publication SW-846 Third Edition (November 1986)
including updates I, II, IIA, IIB and III, as incorporated by reference in
¤60.17(i). For the purposes of a source test that consists of three sample
runs, modify those requirements to include the following: two instrument check
standard runs, two calibration blank runs, one interference check sample at the
beginning of the analysis (analyze by Method of Standard Additions unless
within 25 percent), one quality control sample to check the accuracy of the calibration
standards (required to be within 25 percent of calibration), and one duplicate
analysis (required to be within 20 percent of average or repeat all analyses).
9.2.2 Direct
Aspiration AAS and/or GFAAS Analysis for Sb, As, Ba, Be, Cd, Cu, Cr, Co, Pb, Ni,
Mn, Hg, P, Se, Ag, Tl, and Zn. Analyze all samples in duplicate. Perform a
matrix spike on at least one front-half sample and one back-half sample, or one
combined sample. If recoveries of less than 75 percent or greater than 125
percent are obtained for the matrix spike, analyze each sample by the Method of
Standard Additions. Analyze a quality control sample to check the accuracy of
the calibration standards. If the results are not within 20 percent, repeat the
calibration.
9.2.3 CVAAS Analysis
for Hg. Analyze all samples in duplicate. Analyze a quality control sample to
check the accuracy of the calibration standards (if not within 15 percent,
repeat calibration). Perform a matrix spike on one sample (if not within 25
percent, analyze all samples by the Method of Standard Additions). Additional
information on quality control can be obtained from Method 7470 in EPA
Publication SW-846 Third Edition (November 1986) including updates I, II, IIA,
IIB and III, as incorporated by reference in ¤60.17(i),or in Standard Methods
for Water and Wastewater Method 303F.
10.0 Calibration and Standardization.
NOTE: Maintain a laboratory log of all calibrations.
10.1 Sampling Train Calibration.
Calibrate the
sampling train components according to the indicated sections of Method 5:
Probe Nozzle (Section 10.1); Pitot Tube (Section 10.2); Metering System (Section 10.3); Probe Heater (Section 10.4); Temperature Sensors (Section 10.5); Leak-Check of the Metering
System (Section 8.4.1); and Barometer (Section 10.6).
10.2 Inductively Coupled Argon Plasma Spectrometer Calibration.
Prepare standards as
outlined in Section 7.5. Profile and calibrate the
instrument according to the manufacturer's recommended procedures using those
standards. Check the calibration once per hour. If the instrument does not
reproduce the standard concentrations within 10 percent, perform the complete
calibration procedures. Perform ICP-MS analysis by following Method 6020 in EPA
Publication SW-846 Third Edition (November 1986) including updates I, II, IIA, IIB
and III, as incorporated by reference in ¤60.17(i).
10.3 Atomic Absorption Spectrometer Ð Direct Aspiration AAS, GFAAS, and CVAAS analyses.
Prepare the standards
as outlined in Section 7.5 and use them to calibrate the spectrometer.
Calibration procedures are also outlined in the EPA methods referred to in Table 29-2 and in Method 7470 in EPA Publication SW-846
Third Edition (November 1986) including updates I, II, IIA, IIB and III, as
incorporated by reference in ¤60.17(i), or in Standard Methods for Water and
Wastewater Method 303F (for Hg). Run each standard curve in duplicate and use
the mean values to calculate the calibration line. Recalibrate the instrument
approximately once every 10 to 12 samples.
11.0 Analytical Procedure.
11.1 Sample Analysis.
For each sampling
train sample run, seven individual analytical samples are generated; two for
all desired metals except Hg, and five for Hg. A schematic identifying each sample
container and the prescribed analytical preparation and analysis scheme is
shown in Figure 29-3. The first two analytical samples,
labeled Analytical Fractions 1A and 1B, consist of the digested samples from
the front-half of the train. Analytical Fraction 1A is for ICAP, ICP-MS or AAS
analysis as described in Sections 11.1.1 and 11.1.2, respectively. Analytical
Fraction 1B is for front-half Hg analysis as described in Section 11.1.3. The
contents of the back half of the train are used to prepare the third through
seventh analytical samples. The third and fourth analytical samples, labeled
Analytical Fractions 2A and 2B, contain the samples from the moisture removal
impinger No. 1, if used, and HNO3/H2O2 impingers Nos. 2 and 3. Analytical Fraction 2A
is for ICAP, ICP-MS or AAS analysis for target metals, except Hg. Analytical
Fraction 2B is for analysis for Hg. The fifth through seventh analytical
samples, labeled Analytical Fractions 3A, 3B, and 3C, consist of the impinger
contents and rinses from the empty impinger No. 4 and the H2SO4/KMnO4 Impingers
Nos. 5 and 6. These analytical samples are for analysis for Hg as described in
Section 11.1.3. The total back-half Hg catch is determined from the sum of
Analytical Fractions 2B, 3A, 3B, and 3C. Analytical Fractions 1A and 2A can be
combined proportionally prior to analysis.
11.1.1 ICAP and ICP-MS Analysis.
Analyze Analytical
Fractions 1A and 2A by ICAP using Method 6010 or Method 200.7 (40 CFR 136,
Appendix C). Calibrate the ICAP, and set up an analysis program as described in
Method 6010 or Method 200.7. Follow the quality control procedures described in
Section 9.2.1. Recommended wavelengths for analysis are as shown in Table 29-2. These wavelengths represent the best
combination of specificity and potential detection limit. Other wavelengths may
be substituted if they can provide the needed specificity and detection limit,
and are treated with the same corrective techniques for spectral interference.
Initially, analyze all samples for the target metals (except Hg) plus Fe and
Al. If Fe and Al are present, the sample might have to be diluted so that each
of these elements is at a concentration of less than 50 ppm so as to reduce
their spectral interferences on As, Cd, Cr, and Pb. Perform ICP-MS analysis by
following Method 6020 in EPA Publication SW-846 Third Edition (November 1986)
including updates I, II, IIA, IIB and III, as incorporated by reference in
¤60.17(i).
NOTE: When analyzing samples in a HF matrix, an
alumina torch should be used; since all front-half samples will contain HF, use
an alumina torch.
11.1.2 AAS by Direct Aspiration and/or GFAAS.
If analysis of metals
in Analytical Fractions 1A and 2A by using GFAAS or direct aspiration AAS is
needed, use Table 29-3 to determine which techniques and
procedures to apply for each target metal. Use Table 29-3, if necessary, to
determine techniques for minimization of interferences. Calibrate the
instrument according to Section 10.3 and follow the quality control procedures
specified in Section 9.2.2.
11.1.3 CVAAS Hg analysis.
Analyze Analytical
Fractions 1B, 2B, 3A, 3B, and 3C separately for Hg using CVAAS following the
method outlined in Method 7470 in EPA Publication SW-846 Third Edition
(November 1986) including updates I, II, IIA, IIB and III, as incorporated by
reference in ¤60.17(i), or in Standard Methods for Water and Wastewater
Analysis, 15th Edition, Method 303F, or, optionally using NOTE No. 2 at the end of this section. Set up the
calibration curve (zero to 1000 ng) as described in Method 7470 or similar to
Method 303F using 300-ml BOD bottles instead of Erlenmeyers. Perform the
following for each Hg analysis. From each original sample, select and record an
aliquot in the size range from 1 ml to 10 ml. If no prior knowledge of the
expected amount of Hg in the sample exists, a 5 ml aliquot is suggested for the
first dilution to 100 ml (see NOTE No. 1 at end of this section). The total amount of Hg in the aliquot
shall be less than 1 µg and within the range (zero to 1000 ng) of the
calibration curve. Place the sample aliquot into a separate 300-ml BOD bottle,
and add enough water to make a total volume of 100 ml. Next add to it
sequentially the sample digestion solutions and perform the sample preparation
described in the procedures of Method 7470 or Method 303F. (See NOTE No. 2 at the end of this section). If the maximum
readings are off-scale (because Hg in the aliquot exceeded the calibration
range; including the situation where only a 1-ml aliquot of the original sample
was digested), then dilute the original sample (or a portion of it) with 0.15
percent HNO3 (1.5 ml concentrated HNO3 per liter aqueous solution) so that when a 1- to 10-ml aliquot of
the "0.15 HNO3
percent dilution of the original
sample" is digested and analyzed by the procedures described above, it
will yield an analysis within the range of the calibration curve.
NOTE No. 1: When Hg levels in the sample fractions are
below the in-stack detection limit given in Table 29-1, select a 10 ml aliquot
for digestion and analysis as described.
NOTE No. 2: Optionally, Hg can be analyzed by using the
CVAAS analytical procedures given by some instrument manufacturer's directions.
These include calibration and quality control procedures for the Leeman Model
PS200, the Perkin Elmer FIAS systems, and similar models, if available, of
other instrument manufacturers. For digestion and analyses by these
instruments, perform the following two steps:
(1), Digest the
sample aliquot through the addition of the aqueous hydroxylamine
hydrochloride/sodium chloride solution the same as described in this section:
(The Leeman, Perkin Elmer, and similar instruments described in this note add
automatically the necessary stannous chloride solution during the automated
analysis of Hg.);
(2), Upon completion
of the digestion described in (1), analyze the sample according to the
instrument manufacturer's directions. This approach allows multiple (including
duplicate) automated analyses of a digested sample aliquot.
12.0 Data Analysis and Calculations.
12.1 Nomenclature.
A = Analytical
detection limit, µg/ml.
B = Liquid volume of
digested sample prior to aliquoting for analysis, ml.
C = Stack sample gas
volume, dsm3.
Ca1 = Concentration of metal in Analytical Fraction
1A as read from the standard curve, µg/ml.
Ca2 = Concentration of metal in Analytical Fraction
2A as read from the standard curve, (µg/ml).
Cs = Concentration of a metal in the stack gas, mg/dscm.
D = In-stack
detection limit, µg/m3.
Fa = Aliquot factor, volume of Sample Fraction 2 divided by volume of
Sample Fraction 2A (see Section 8.3.4.)
Fd = Dilution factor (Fd = the inverse of the
fractional portion of the concentrated sample in the solution actually used in
the instrument to produce the reading Ca1. For
example, if a 2 ml aliquot of Analytical Fraction 1A is diluted to 10 ml to
place it in the calibration range, Fd = 5).
Hgbh = Total mass of Hg collected in the back half of
the sampling train, µg.
Hgbh2 = Total mass of Hg collected in Sample Fraction
2, µg.
Hgbh3(A,B,C) = Total mass of Hg collected separately in
Fraction 3A, 3B, or 3C, µg.
Hgbhb = Blank correction value for mass of Hg detected
in back-half field reagent blanks, µg.
Hgfh = Total mass of Hg collected in the front half of
the sampling train (Sample Fraction 1), µg.
Hgfhb = Blank correction value for mass of Hg detected
in front-half field reagent blank, µg.
Hgt = Total mass of Hg collected in the sampling train, µg.
Mbh = Total mass of each metal (except Hg) collected
in the back half of the sampling train (Sample Fraction 2), µg.
Mbhb = Blank correction value for mass of metal
detected in back-half field reagent blank, µg.
Mfh = Total mass of each metal (except Hg) collected
in the front half of the sampling train (Sample Fraction 1), µg.
Mfhb = Blank correction value for mass of metal
detected in front-half field reagent blank, µg.
Mt = Total mass of each metal (separately stated for each metal)
collected in the sampling train, µg.
Mt = Total mass of that metal collected in the sampling train, µg;
(substitute Hgt for Mt
for the Hg
calculation).
Qbh2 = Quantity of Hg, µg, TOTAL in the ALIQUOT of
Analytical Fraction 2B selected for digestion and analysis .
NOTE: For example, if a 10 ml aliquot of Analytical
Fraction 2B is taken and digested and analyzed (according to Section 11.1.3 and
its NOTES Nos. 1 and 2),
then calculate and use the total amount of Hg in the 10 ml aliquot for Qbh2.
Qbh3(A,B,C) = Quantity of Hg, µg, TOTAL, separately, in the ALIQUOT
of Analytical Fraction 3A, 3B, or 3C selected for digestion and analysis (see NOTES
in Sections 12.7.1 and 12.7.2
describing the quantity "Q" and calculate similarly).
Qfh = Quantity of Hg, µg, TOTAL in the ALIQUOT of
Analytical Fraction 1B selected for digestion and analysis .
NOTE: For example, if a 10 ml aliquot of Analytical
Fraction 1B is taken and digested and analyzed (according to Section 11.1.3 and
its NOTES Nos. 1 and 2), then calculate
and use the total
amount of Hg in the 10 ml aliquot for Qfh.
Va = Total volume of digested sample solution (Analytical Fraction
2A), ml (see Section 8.3.4.1 or 8.3.4.2, as applicable).
Vf1B = Volume of aliquot of Analytical Fraction 1B
analyzed, ml.
NOTE: For example, if a 1 ml aliquot of Analytical Fraction
1B was diluted to 50 ml with 0.15 percent HNO3 as
described in Section 11.1.3 to bring it into the proper analytical range, and
then 1 ml of that 50-ml was digested according to Section 11.1.3 and analyzed,
Vf1B would be 0.02 ml.
Vf2B = Volume of Analytical Fraction 2B analyzed, ml.
NOTE: For example, if 1 ml of Analytical Fraction 2B
was diluted to 10 ml with 0.15 percent HNO3 as
described in Section 11.1.3 to bring it into the proper analytical range, and
then 5 ml of that 10 ml was analyzed, Vf2B would
be 0.5 ml.
Vf3(A,B,C) = Volume, separately, of Analytical Fraction 3A,
3B, or 3C analyzed, ml (see previous notes in Sections 12.7.1 and 12.7.2,
describing the quantity "V" and calculate similarly).
Vm(std) = Volume of gas sample as measured by the dry
gas meter, corrected to dry standard conditions, dscm.
Vsoln,1 = Total volume of digested sample solution
(Analytical Fraction 1), ml.
Vsoln,1 = Total volume of Analytical Fraction 1, ml.
Vsoln,2 = Total volume of Sample Fraction 2, ml.
Vsoln,3(A,B,C)= Total volume, separately, of Analytical
Fraction 3A, 3B, or 3C, ml.
K4 = 10-3 mg/µg.
12.2 Dry Gas Volume.
Using the data from this test, calculate Vm(std), the
dry gas sample volume at standard conditions as outlined in Section 12.3 of Method 5.
12.3 Volume of Water
Vapor and Moisture Content. Using the total volume of condensate collected
during the source sampling, calculate the volume of water vapor Vw(std) and the moisture content Bws of the stack gas. Use Equations 5-2 and 5-3 of Method 5.
12.4 Stack Gas
Velocity. Using the data from this test and Equation
2-9 of Method 2, calculate the average stack gas velocity.
12.5 In-Stack
Detection Limits. Calculate the in-stack method detection limits shown in Table
29-4 using the conditions described in Section 13.3.1 as follows:

12.6 Metals (Except
Hg) in Source Sample.
12.6.1 Analytical Fraction
1A, Front-Half, Metals (except Hg). Calculate separately the amount of each
metal collected in Sample Fraction 1 of the sampling train using the following
equation:
![]()
NOTE: If Analytical Fractions 1A and 2A are
combined, use proportional aliquots. Then make appropriate changes in Equations
29-2 through 29-4 to reflect this approach.
12.6.2 Analytical
Fraction 2A, Back-Half, Metals (except Hg). Calculate separately the amount of
each metal collected in Fraction 2 of the sampling train using the following
equation:
12.6.3 Total Train,
Metals (except Hg). Calculate the total amount of each of the quantified metals
collected in the sampling train as follows:
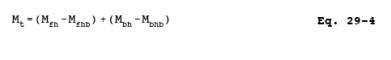
NOTE: If the measured blank value for the front half
(Mfhb) is in the range 0.0 to "A" µg [where
"A" µg equals the value determined by multiplying 1.4 µg/in.2 times the actual area in in.2 of the
sample filter], use Mfhb
to correct the emission sample
value (Mfh); if Mfhb exceeds
"A" µg, use the greater of I or II:
I. "A" µg.
II. the lesser of (a)
Mfhb, or (b) 5 percent of Mfh. If the measured blank value for the back half (Mbhb) is in the range 0.0 to 1 µg, use Mbhb to correct the emission sample value (Mbh); if Mbhb
exceeds 1 µg, use the greater of I
or II:
I. 1 µg.
II. the lesser of (a)
Mbhb, or (b) 5 percent of Mbh.
12.7 Hg in Source
Sample.
12.7.1 Analytical
Fraction 1B; Front-Half Hg. Calculate the amount of Hg collected in the
front-half, Sample Fraction 1, of the sampling train by using Equation 29-5:

12.7.2 Analytical
Fractions 2B, 3A, 3B, and 3C; Back Half Hg.
12.7.2.1 Calculate
the amount of Hg collected in Sample Fraction 2 by using Equation 29-6:

12.7.2.2 Calculate
each of the back-half Hg values for Analytical Fractions 3A, 3B, and 3C by
using Equation 29-7:
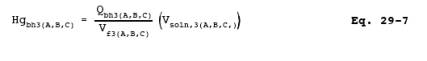
12.7.2.3 Calculate
the total amount of Hg collected in the back-half of the sampling train by
using Equation 29-8:
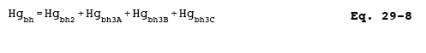
12.7.3 Total Train Hg
Catch. Calculate the total amount of Hg collected in the sampling train by
using Equation 29-9:
![]()
NOTE: If the total of the measured blank values (Hgfhb + Hgbhb) is in
the range of 0.0 to 0.6 µg, then use the total to correct the sample value (Hgfh + Hgbh); if it
exceeds 0.6 µg, use the greater of I. or II:
I. 0.6 µg.
II. The lesser of (a)
(Hgfhb + Hgbhb), or
(b) 5 percent of the sample value (Hgfh + Hgbh).
12.8 Individual Metal
Concentrations in Stack Gas. Calculate the concentration of each metal in the
stack gas (dry basis, adjusted to standard conditions) by using Equation 29-10:

12.9 Isokinetic
Variation and Acceptable Results. Same as Method
5, Sections 12.11 and 12.12, respectively.
13.0 Method Performance.
13.1 Range.
For the analysis
described and for similar analyses, the ICAP response is linear over several
orders of magnitude. Samples containing metal concentrations in the nanograms
per ml (ng/ml) to micrograms per ml (µg/ml) range in the final analytical
solution can be analyzed using this method. Samples containing greater than approximately
50 µg/ml As, Cr, or Pb should be diluted to that level or lower for final
analysis. Samples containing greater than approximately 20 µg/ml of Cd should
be diluted to that level before analysis.
13.2 Analytical Detection Limits.
NOTE: See Section 13.3 for the description of
in-stack detection limits.
13.2.1 ICAP
analytical detection limits for the sample solutions (based on SW-846, Method
6010) are approximately as follows: Sb (32 ng/ml), As (53 ng/ml), Ba (2 ng/ml),
Be (0.3 ng/ml), Cd (4 ng/ml), Cr (7 ng/ml), Co (7 ng/ml), Cu (6 ng/ml), Pb (42
ng/ml), Mn (2 ng/ml), Ni (15 ng/ml), P (75 ng/ml), Se (75 ng/ml), Ag (7 ng/ml),
Tl (40 ng/ml), and Zn (2 ng/ml). ICP-MS analytical detection limits (based on
SW-846, Method 6020) are lower generally by a factor of ten or more. Be is
lower by a factor of three. The actual sample analytical detection limits are
sample dependent and may vary due to the sample matrix.
13.2.2 The analytical
detection limits for analysis by direct aspiration AAS (based on SW-846, Method
7000 series) are approximately as follow: Sb (200 ng/ml), As (2 ng/ml), Ba (100
ng/ml), Be (5 ng/ml), Cd (5 ng/ml), Cr (50 ng/ml), Co (50 ng/ml), Cu (20
ng/ml), Pb (100 ng/ml), Mn (10 ng/ml), Ni (40 ng/ml), Se (2 ng/ml), Ag (10
ng/ml), Tl (100 ng/ml), and Zn (5 ng/ml).
13.2.3 The detection
limit for Hg by CVAAS (on the resultant volume of the digestion of the aliquots
taken for Hg analyses) can be approximately 0.02 to 0.2 ng/ml, depending upon
the type of CVAAS analytical instrument used.
13.2.4 The use of
GFAAS can enhance the detection limits compared to direct aspiration AAS as
follows: Sb (3 ng/ml), As (1 ng/ml), Be (0.2 ng/ml), Cd (0.1 ng/ml), Cr (1
ng/ml), Co (1 ng/ml), Pb (1 ng/ml), Se (2 ng/ml), and Tl (1 ng/ml).
13.3 In-stack Detection Limits.
13.3.1 For test
planning purposes in-stack detection limits can be developed by using the
following information: (1) the procedures described in this method, (2) the
analytical detection limits described in Section 13.2 and in SW-846, (3) the normal
volumes of 300 ml (Analytical Fraction 1) for the front-half and 150 ml
(Analytical Fraction 2A) for the back-half samples, and (4) a stack gas sample
volume of 1.25 m3. The resultant in-stack method detection limits
for the above set of conditions are presented in Table 29-1 and were calculated
by using Eq. 29-1 shown in Section 12.5.
13.3.2 To ensure
optimum precision/resolution in the analyses, the target concentrations of
metals in the analytical solutions should be at least ten times their respective
analytical detection limits. Under certain conditions, and with greater care in
the analytical procedure, these concentrations can be as low as approximately
three times the respective analytical detection limits without seriously
impairing the precision of the analyses. On at least one sample run in the
source test, and for each metal analyzed, perform either repetitive analyses,
Method of Standard Additions, serial dilution, or matrix spike addition, etc.,
to document the quality of the data.
13.3.3 Actual
in-stack method detection limits are based on actual source sampling parameters
and analytical results as described above. If required, the method in-stack
detection limits can be improved over those shown in Table 29-1 for a specific
test by either increasing the sampled stack gas volume, reducing the total
volume of the digested samples, improving the analytical detection limits, or
any combination of the three. For extremely low levels of Hg only, the aliquot
size selected for digestion and analysis can be increased to as much as 10 ml,
thus improving the in-stack detection limit by a factor of ten compared to a 1
ml aliquot size.
13.3.3.1 A nominal
one hour sampling run will collect a stack gas sampling volume of about 1.25 m3. If the sampling time is increased to four hours and 5 m3 are collected, the in-stack method detection limits would be
improved by a factor of four compared to the values shown in Table 29-1.
13.3.3.2 The in-stack
detection limits assume that all of the sample is digested and the final liquid
volumes for analysis are the normal values of 300 ml for Analytical Fraction 1,
and 150 ml for Analytical Fraction 2A. If the volume of Analytical Fraction 1
is reduced from 300 to 30 ml, the in-stack detection limits for that fraction
of the sample would be improved by a factor of ten. If the volume of Analytical
Fraction 2A is reduced from 150 to 25 ml, the in-stack detection limits for
that fraction of the sample would be improved by a factor of six. Matrix effect
checks are necessary on sample analyses and typically are of much greater
significance for samples that have been concentrated to less than the normal
original sample volume. Reduction of Analytical Fractions 1 and 2A to volumes
of less than 30 and 25 ml, respectively, could interfere with the redissolving
of the residue and could increase interference by other compounds to an
intolerable level.
13.3.3.3 When both of
the modifications described in Sections 13.3.3.1 and 13.3.3.2 are used
simultaneously on one sample, the resultant improvements are multiplicative.
For example, an increase in stack gas volume by a factor of four and a
reduction in the total liquid sample digested volume of both Analytical
Fractions 1 and 2A by a factor of six would result in an improvement by a
factor of twenty-four of the in-stack method detection limit.
13.4 Precision.
The precision
(relative standard deviation) for each metal detected in a method development
test performed at a sewage sludge incinerator were found to be as follows:
Sb (12.7 percent), As
(13.5 percent), Ba (20.6 percent),
Cd (11.5 percent), Cr
(11.2 percent), Cu (11.5 percent),
Pb (11.6 percent), P
(14.6 percent), Se (15.3 percent),
Tl (12.3 percent),
and Zn (11.8 percent).
The precision for Ni
was 7.7 percent for another test conducted at a source simulator. Be, Mn, and
Ag were not detected in the tests. However, based on the analytical detection
limits of the ICAP for these metals, their precisions could be similar to those
for the other metals when detected at similar levels.
14.0 Pollution Prevention. [Reserved]
15.0 Waste Management. [Reserved]
16.0 References.
1. Method 303F in
Standard Methods for the Examination of Water Wastewater, 15th Edition, 1980.
Available from the American Public Health Association, 1015 18th Street N.W.,
Washington, D.C. 20036.
2. EPA Methods 6010,
6020, 7000, 7041, 7060, 7131, 7421, 7470, 7740, and 7841, Test Methods for
Evaluating Solid Waste: Physical/Chemical Methods. SW-846, Third Edition,
November 1986, with updates I, II, IIA, IIB and III. Office of Solid Waste and
Emergency Response, U. S. Environmental Protection Agency, Washington, D.C.
20460.
3. EPA Method 200.7,
Code of Federal Regulations, Title 40, Part 136, Appendix C. July 1, 1987.
4. EPA Methods 1
through 5, Code of Federal Regulations, Title 40, Part 60, Appendix A, July 1,
1991.
5. EPA Method 101A,
Code of Federal Regulations, Title 40, Part 61, Appendix B, July 1, 1991.
17.0 Tables, Diagrams, Flowcharts, and Validation Data.
TABLE 29-1. IN-STACK METHOD DETECTION LIMITS
(µg/m3) FOR THE FRONT-HALF, THE BACK-HALF, AND THE
TOTAL SAMPLING TRAIN USING ICAP, GFAAS, AND CVAAS.
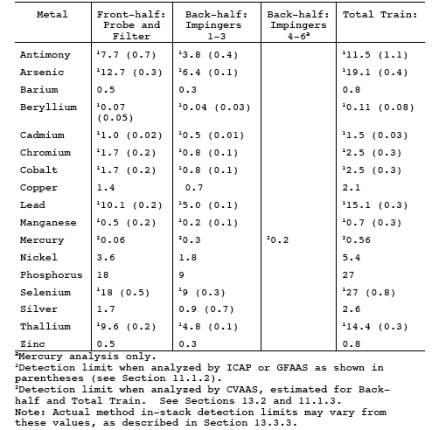
TABLE 29-2. RECOMMENDED WAVELENGTHS FOR ICAP
ANALYSIS
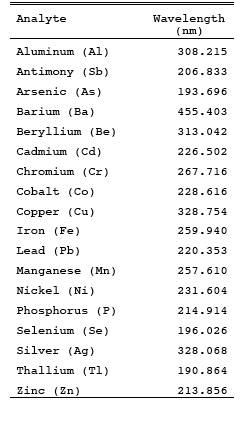
TABLE 29-3. APPLICABLE TECHNIQUES, METHODS AND MINIMIZATION OF INTERFERENCES FOR AAS ANALYSIS.
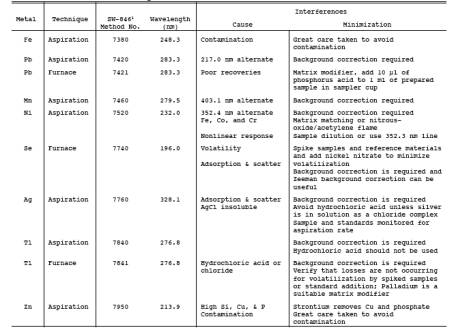
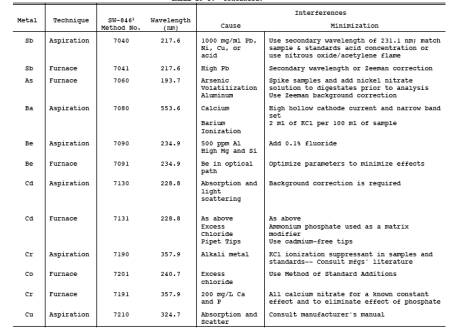
1Refer to EPA publication SW-846 (Reference 2 in
Section 16.0).
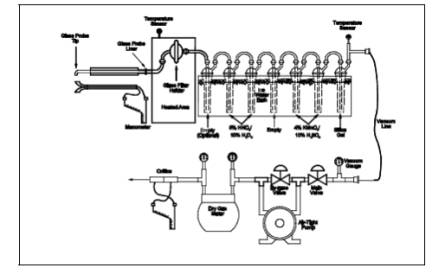
Figure
29-2a. Sample Recovery Scheme.
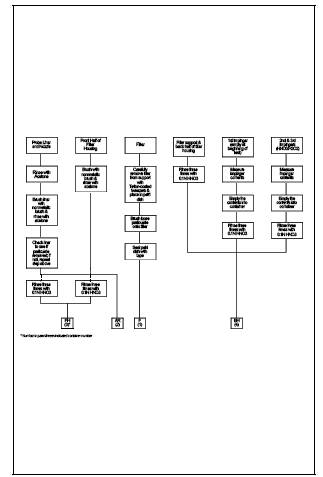
Figure
29-2b. Sample Recovery Scheme.
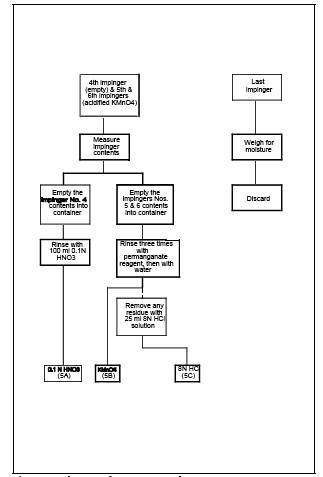
Figure
29-3. Sample Preparation and Analysis Scheme.