METHOD 101A - DETERMINATION OF PARTICULATE AND GASEOUS MERCURY
EMISSIONS FROM SEWAGE SLUDGE INCINERATORS
NOTE: This method does not include all of the
specifications (e.g., equipment
and supplies) and procedures (e.g.,
sampling and analytical) essential to its performance. Some material is
incorporated by reference from methods in Appendix A to 40 CFR Part 60 and in
this part. Therefore, to obtain reliable results, persons using this method
should also have a thorough knowledge of at least the following additional test
methods: Methods 1, Method
2, Method 3, and Method
5 of Part 60 (Appendix A), and Method 101
Part 61 (Appendix B).
6.1 Sample Collection
and Sample Recovery.
7.1 Sample Collection
and Recovery.
8.0 Sample Collection,
Preservation, Transport, and Storage.
8.1 Preliminary
Determinations.
8.2 Preparation of
Sampling Train.
10.0 Calibration and
Standardization.
12.0 Data Analysis and
Calculations.
14.0 Pollution
Prevention. [Reserved]
15.0 Waste Management.
[Reserved]
17.0 Tables, Diagrams, Flowcharts and Validation Data. [Reserved]
1.0 Scope and Application.
1.1 Analytes.

1.2 Applicability.
This method is
applicable for the determination of Hg emissions from sewage sludge
incinerators and other sources as specified in an applicable subpart of the
regulations.
1.3 Data Quality Objectives.
Adherence to the
requirements of this method will enhance the quality of the data obtained from
air pollutant sampling methods.
2.0 Summary of Method.
2.1 Particulate and
gaseous Hg emissions are withdrawn isokinetically from the source and are
collected in acidic potassium permanganate (KMnO4) solution.
The Hg collected (in the mercuric form) is reduced to elemental Hg, which is
then aerated from the solution into an optical cell and measured by atomic
absorption spectrophotometry.
3.0 Definitions. [Reserved]
4.0 Interferences.
4.1 Sample Collection.
Excessive oxidizable organic matter in the stack gas prematurely depletes the
KMnO4 solution and thereby prevents further collection
of Hg.
4.2 Analysis.
Condensation of water vapor on the optical cell windows causes a positive
interference.
5.0 Safety.
5.1 Disclaimer.
This method may
involve hazardous materials, operations, and equipment. This test method may
not address all of the safety problems associated with its use. It is the
responsibility of the user of this test method to establish appropriate safety
and health practices and determine the applicability of regulatory limitations
prior to performing this test method.
5.2 Corrosive Reagents.
The following
reagents are hazardous. Personal protective equipment and safe procedures are
useful in preventing chemical splashes. If contact occurs, immediately flush
with copious amounts of water for at least 15 minutes. Remove clothing under
shower and decontaminate. Treat residual chemical burns as thermal burns.
5.2.1 Hydrochloric
Acid (HCl). Highly toxic. Vapors are highly irritating to eyes, skin, nose, and
lungs, causing severe damage. May cause bronchitis, pneumonia, or edema of
lungs. Exposure to concentrations of 0.13 to 0.2 percent can be lethal to
humans in a few minutes. Provide ventilation to limit exposure. Reacts with
metals, producing hydrogen gas.
5.2.2 Nitric Acid
(HNO3). Highly corrosive to eyes, skin, nose, and
lungs. Vapors cause bronchitis, pneumonia, or edema of lungs. Reaction to
inhalation may be delayed as long as 30 hours and still be fatal. Provide
ventilation to limit exposure. Strong oxidizer. Hazardous reaction may occur
with organic materials such as solvents.
5.2.3 Sulfuric acid
(H2SO4). Rapidly
destructive to body tissue. Will cause third degree burns. Eye damage may
result in blindness. Inhalation may be fatal from spasm of the larynx, usually
within 30 minutes. May cause lung tissue damage with edema. 3 mg/m3 will cause lung damage in uninitiated. 1 mg/m3 for 8 hours will cause lung damage or, in higher concentrations,
death. Provide ventilation to limit inhalation. Reacts violently with metals
and organics.
5.3 Chlorine Evolution.
Hydrochloric acid
reacts with KMnO4 to liberate chlorine gas. Although this is a
minimal concern when small quantities of HCl (5-10 ml) are used in the impinger
rinse, a potential safety hazard may still exist. At sources that emit higher
concentrations of oxidizable materials (e.g., power plants), more HCl may be required to remove
the larger amounts of brown deposit formed in the impingers. In such cases, the
potential safety hazards due to sample container pressurization are greater,
because of the larger volume of HCl rinse added to the recovered sample. These
hazards are eliminated by storing and analyzing the HCl impinger wash
separately from the permanganate impinger sample.
6.0 Equipment and Supplies.
6.1 Sample Collection and Sample Recovery.
Same as Method 101, Sections 6.1 and 6.2,
respectively, with the following exceptions:
6.1.1 Probe Liner.
Same as in Method 101, Section 6.1.2, except that if a filter is used ahead of
the impingers, the probe heating system must be used to minimize the
condensation of gaseous Hg.
6.1.2 Filter Holder
(Optional). Borosilicate glass with a rigid stainless-steel wire-screen filter
support (do not use glass frit supports) and a silicone rubber or Teflon
gasket, designed to provide a positive seal against leakage from outside or
around the filter. The filter holder must be equipped with a filter heating
system capable of maintaining a temperature around the filter holder of 120 ±
14 ¼C (248 ± 25 ¼F) during sampling to minimize both water and gaseous Hg
condensation. A filter may also be used in cases where the stream contains
large quantities of particulate matter.
6.2 Sample Analysis.
Same as Method 101, Section 6.3, with the following
additions and exceptions:
6.2.1 Volumetric
Pipets. Class A; 1-, 2-, 3-, 4-, 5-, 10-, and 20-ml.
6.2.2 Graduated
Cylinder. 25-ml.
6.2.3 Steam Bath.
6.2.4 Atomic
Absorption Spectrophotometer or Equivalent. Any atomic absorption unit with an
open sample presentation area in which to mount the optical cell is suitable.
Instrument settings recommended by the particular manufacturer should be
followed. Instruments designed specifically for the measurement of mercury
using the cold-vapor technique are commercially available and may be
substituted for the atomic absorption spectrophotometer.
6.2.5 Optical Cell.
Alternatively, a heat lamp mounted above the cell or a moisture trap installed
upstream of the cell may be used.
6.2.6 Aeration Cell.
Alternatively, aeration cells available with commercial cold vapor instrumentation
may be used.
6.2.7 Aeration Gas
Cylinder. Nitrogen, argon, or dry, Hg-free air, equipped with a single-stage
regulator. Alternatively, aeration may be provided by a peristaltic metering
pump. If a commercial cold vapor instrument is used, follow the manufacturer's
recommendations.
7.0 Reagents and Standards.
Unless otherwise
indicated, it is intended that all reagents conform to the specifications
established by the Committee on Analytical Reagents of the American Chemical
Society, where such specifications are available; otherwise, use the best
available grade.
7.1 Sample Collection and Recovery.
The following
reagents are required for sample collection and recovery:
7.1.1 Water.
Deionized distilled, to conform to ASTM D 1193-77 or 91 Type 1. If high
concentrations of organic matter are not expected to be present, the analyst
may eliminate the KMnO4
test for oxidizable organic matter.
Use this water in all dilutions and solution preparations.
7.1.2 Nitric Acid, 50
Percent (V/V). Mix equal volumes of concentrated HNO3 and water, being careful to add the acid to the water slowly.
7.1.3 Silica Gel.
Indicating type, 6 to 16 mesh. If previously used, dry at 175 ¼C (350 ¼F) for 2
hours. New silica gel may be used as received.
7.1.4 Filter
(Optional). Glass fiber filter, without organic binder, exhibiting at least
99.95 percent efficiency on 0.3-µm dioctyl phthalate smoke particles. The
filter in cases where the gas stream contains large quantities of particulate
matter, but blank filters should be analyzed for Hg content.
7.1.5 Sulfuric Acid,
10 Percent (V/V). Carefully add and mix 100 ml of concentrated H2SO4 to 900 ml of water.
7.1.6 Absorbing
Solution, 4 Percent KMnO4
(W/V). Prepare fresh daily.
Dissolve 40 g of KMnO4
in sufficient 10 percent H2SO4 to make 1 liter. Prepare and store in glass
bottles to prevent degradation.
7.1.7 Hydrochloric
Acid, 8 N. Carefully add and mix 67 ml of concentrated HCl to 33 ml of water.
7.2 Sample Analysis.
The following
reagents and standards are required for sample analysis:
7.2.1 Water. Same as
in Section 7.1.1.
7.2.2 Tin (II)
Solution. Prepare fresh daily, and keep sealed when not being used. Completely
dissolve 20 g of tin (II) chloride [or 25 g of tin (II) sulfate] crystals (Baker
Analyzed reagent grade or any other brand that will give a clear solution) in
25 ml of concentrated HCl. Dilute to 250 ml with water. Do not substitute HNO3, H2SO4, or other
strong acids for the HCl.
7.2.3 Sodium
Chloride-Hydroxylamine Solution. Dissolve 12 g of sodium chloride and 12 g of
hydroxylamine sulfate (or 12 g of hydroxylamine hydrochloride) in water and
dilute to 100 ml.
7.2.4 Hydrochloric
Acid, 8 N. Same as Section 7.1.7
7.2.5 Nitric Acid, 15
Percent (V/V). Carefully add 15 ml HNO3 to 85 ml
of water.
7.2.6 Antifoam B
Silicon Emulsion. J.T. Baker Company (or equivalent).
7.2.7 Mercury Stock
Solution, 1 mg Hg/ml. Prepare and store all Hg standard solutions in
borosilicate glass containers. Completely dissolve 0.1354 g of Hg (II) chloride
in 75 ml of water. Add 10 ml of concentrated HNO3,
and adjust the volume to exactly 100 ml with water. Mix thoroughly. This
solution is stable for at least one month.
7.2.8 Intermediate Hg
Standard Solution, 10 µg/ml. Prepare fresh weekly. Pipet 5.0 ml of the Hg stock
solution (Section 7.2.7) into a 500 ml volumetric flask, and add 20 ml of 15
percent HNO3 solution. Adjust the volume to exactly 500 ml
with water. Thoroughly mix the solution.
7.2.9 Working Hg
Standard Solution, 200 ng Hg/ml. Prepare fresh daily. Pipet 5.0 ml from the
"Intermediate Hg Standard Solution" (Section 7.2.8) into a 250-ml
volumetric flask. Add 5 ml of 4 percent KMnO4 absorbing
solution and 5 ml of 15 percent HNO3. Adjust
the volume to exactly 250 ml with water. Mix thoroughly.
7.2.10 Potassium
Permanganate, 5 Percent (W/V). Dissolve 5 g of KMnO4 in water and dilute to 100 ml.
7.2.11 Filter.
Whatman No. 40, or equivalent.
8.0 Sample Collection, Preservation, Transport, and Storage.
Same as Method 101, Section 8.0, with the exception of
the following:
8.1 Preliminary Determinations.
Same as Method 101, Section 8.2, except that the liberation
of free iodine in the first impinger due to high Hg or sulfur dioxide
concentrations is not applicable. In this method, high oxidizable organic
content may make it impossible to sample for the desired minimum time. This
problem is indicated by the complete bleaching of the purple color of the KMnO4 solution. In cases where an excess of water condensation is
encountered, collect two runs to make one sample, or add an extra impinger in
front of the first impinger (also containing acidified KMnO4 solution).
8.2 Preparation of Sampling Train.
Same as Method 101, Section 8.3, with the exception of
the following:
8.2.1 In this method,
clean all the glass components by rinsing with 50 percent HNO3, tap water, 8 N HCl, tap water, and finally with deionized
distilled water. Then place 50 ml of absorbing solution in the first impinger
and 100 ml in each of the second and third impingers.
8.2.2 If a filter is
used, use a pair of tweezers to place the filter in the filter holder. Be sure
to center the filter, and place the gasket in the proper position to prevent
the sample gas stream from bypassing the filter. Check the filter for tears
after assembly is completed. Be sure also to set the filter heating system at the
desired operating temperature after the sampling train has been assembled.
8.3 Sampling Train Operation.
In addition to the
procedure outlined in Method 101, Section 8.5,
maintain a temperature around the filter (if applicable) of 120 ± 14 ¼C (248 ±
25 ¼F).
8.4 Sample Recovery.
Same as Method 101, Section 8.7, with the exception of
the following:
8.4.1 Transfer the probe,
impinger assembly, and (if applicable) filter assembly to the cleanup area.
8.4.2 Treat the
sample as follows:
8.4.2.1 Container No.
1 (Impinger, Probe, and Filter Holder) and, if applicable, Container No. 1A
(HCl rinse).
8.4.2.1.1 Using a graduated
cylinder, measure the liquid in the first three impingers to within 1 ml.
Record the volume of liquid present (e.g., see Figure 5-6 of Method 5). This
information is needed to calculate the moisture content of the effluent gas.
(Use only graduated cylinder and glass storage bottles that have been
pre-cleaned as in Section 8.2.1.) Place the contents of the first three
impingers (four if an extra impinger was added as described in Section 8.1)
into a 1000-ml glass sample bottle labeled Container No. 1.
NOTE: If a filter is used, remove the filter from its
holder as outlined under Section 8.4.3.
8.4.2.1.2 Taking care
that dust on the outside of the probe or other exterior surfaces does not get
into the sample, quantitatively recover the Hg (and any condensate) from the
probe nozzle, probe fitting, probe liner, front half of the filter holder (if
applicable), and impingers as follows: Rinse these components with a total of
400 ml (350 ml if an extra impinger was added as described in Section 8.1) of fresh absorbing solution, carefully
assuring removal of all loose particulate matter from the impingers; add all
washings to the 1000 ml glass sample bottle. To remove any residual brown
deposits on the glassware following the permanganate rinse, rinse with
approximately 100 ml of water, carefully assuring removal of all loose
particulate matter from the impingers. Add this rinse to Container No. 1.
8.4.2.1.3 If no
visible deposits remain after this water rinse, do not rinse with 8 N HCl. If
deposits do remain on the glassware after the water rinse, wash impinger walls
and stems with 25 ml of 8 N HCl, and place the wash in a separate container
labeled Container No. 1A as follows: Place 200 ml of water in a sample
container labeled Container No. 1A. Wash the impinger walls and stem with the
HCl by turning the impinger on its side and rotating it so that the HCl
contacts all inside surfaces. Pour the HCl. wash carefully with stirring into
Container No. 1A.
8.4.2.1.4 After all
washings have been collected in the appropriate sample container(s), tighten
the lid(s) on the container(s) to prevent leakage during shipment to the
laboratory. Mark the height of the fluid level to allow subsequent
determination of whether leakage has occurred during transport. Label each
container to identify its contents clearly.
8.4.3 Container No. 2
(Silica Gel). Same as Method 5, Section
8.7.6.3.
8.4.4 Container No. 3
(Filter). If a filter was used, carefully remove it from the filter holder,
place it in a 100-ml glass sample bottle, and add 20 to 40 ml of absorbing
solution. If it is necessary to fold the filter, be sure that the particulate
cake is inside the fold. Carefully transfer to the 100-ml sample bottle any
particulate matter and filter fibers that adhere to the filter holder gasket by
using a dry Nylon bristle brush and a sharp-edged blade. Seal the container.
Label the container to identify its contents clearly. Mark the height of the
fluid level to allow subsequent determination of whether leakage has occurred
during transport.
8.4.5 Container No. 4
(Filter Blank). If a filter was used, treat an unused filter from the same
filter lot as that used for sampling according to the procedures outlined in
Section 8.4.4.
8.4.6 Container No. 5
(Absorbing Solution Blank). Place 650 ml of 4 percent KMnO4 absorbing solution in a 1000-ml sample bottle. Seal the container.
8.4.7 Container No. 6
(HCl Rinse Blank). Place 200 ml of water in a 1000-ml sample bottle, and add 25
ml of 8 N HCl carefully with stirring. Seal the container. Only one blank
sample per 3 runs is required.
9.0 Quality Control.
9.1 Miscellaneous
Quality Control Measures.
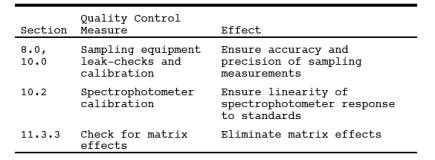
9.2 Volume Metering System
Checks. Same as Method 5, Section 9.2.
10.0 Calibration and Standardization.
Same as Method 101, Section 10.0, with the following
exceptions:
10.1 Optical Cell Heating
System Calibration. Same as in Method 101, Section 10.4, except use a-25 ml
graduated cylinder to add 25 ml of water to the bottle section of the aeration
cell.
10.2
Spectrophotometer and Recorder Calibration.
10.2.1 The Hg
response may be measured by either peak height or peak area.
NOTE: The temperature of the solution affects the rate
at which elemental Hg is released from a solution and, consequently, it affects
the shape of the absorption curve (area) and the point of maximum absorbance
(peak height). To obtain
reproducible results, all solutions must be brought to room temperature before
use.
10.2.2 Set the
spectrophotometer wave length at 253.7 nm, and make certain the optical cell is
at the minimum temperature that will prevent water condensation. Then set the
recorder scale as follows: Using a 25-ml graduated cylinder, add 25 ml of water
to the aeration cell bottle. Add three drops of Antifoam B to the bottle, and
then pipet 5.0 ml of the working Hg standard solution into the aeration cell.
NOTE: Always add the Hg-containing solution to the
aeration cell after the 25 ml of water.
10.2.3 Place a
Teflon-coated stirring bar in the bottle. Add 5 ml of absorbing solution to the
aeration bottle, and mix well. Before attaching the bottle section to the
bubbler section of the aeration cell, make certain that (1) the aeration cell
exit arm stopcock (Figure 101-3 of Method 101)
is closed (so that Hg will not prematurely enter the optical cell when the
reducing agent is being added) and (2) there is no flow through the bubbler. If
conditions (1) and (2) are met, attach the bottle section to the bubbler
section of the aeration cell. Add sodium chloride-hydroxylamine in 1 ml
increments until the solution is colorless. Now add 5 ml of tin (II) solution
to the aeration bottle through the side arm, and immediately stopper the side
arm. Stir the solution for 15 seconds, turn on the recorder, open the aeration
cell exit arm stopcock, and immediately initiate aeration with continued
stirring. Determine the maximum absorbance of the standard, and set this value
to read 90 percent of the recorder full scale.
11.0 Analytical Procedure.
11.1 Sample Loss Check.
Check the liquid
level in each container to see if liquid was lost during transport. If a
noticeable amount of leakage occurred, either void the sample or use methods
subject to the approval of the Administrator to account for the losses.
11.2 Sample Preparation.
Treat sample
containers as follows:
11.2.1 Containers No.
3 and No. 4 (Filter and FilterBlank).
11.2.1.1 If a filter
is used, place the contents, including the filter, of Containers No. 3 and No.
4 in separate 250-ml beakers, and heat the beakers on a steam bath until most of
the liquid has evaporated. Do not heat to dryness. Add 20 ml of concentrated
HNO3 to the beakers, cover them with a watch glass,
and heat on a hot plate at 70 ¼C (160 ¼F) for 2 hours. Remove from the hot
plate.
11.2.1.2 Filter the
solution from digestion of the Container No. 3 contents through Whatman No. 40
filter paper, and save the filtrate for addition to the Container No. 1
filtrate as described in Section 11.2.2. Discard the filter paper.
11.2.1.3 Filter the
solution from digestion of the Container No. 4 contents through Whatman No. 40
filter paper, and save the filtrate for addition to Container No. 5 filtrate as
described in Section 11.2.3 below. Discard the filter paper.
11.2.2 Container No. 1 (Impingers, Probe, and Filter Holder) and,
if applicable, No. 1A (HCl rinse).
11.2.2.1 Filter the
contents of Container No. 1 through Whatman No. 40 filter paper into a 1 liter
volumetric flask to remove the brown manganese dioxide (MnO2) precipitate. Save the filter for digestion of
the brown MnO2 precipitate. Add the sample filtrate from
Container No. 3 to the 1-liter volumetric flask, and dilute to volume with
water. If the combined filtrates are greater than 1000 ml, determine the volume
to the nearest ml and make the appropriate corrections for blank subtractions.
Mix thoroughly. Mark the filtrate as analysis Sample No. A.1 and analyze for Hg
within 48 hr of the filtration step. Place the saved filter, which was used to
remove the brown MnO2
precipitate, into an appropriate
sized container. In a laboratory hood, add 25 ml of 8 N HCl to the filter and
allow to digest for a minimum of 24 hours at room temperature.
11.2.2.2 Filter the
contents of Container 1A through Whatman No. 40 filter paper into a 500-ml
volumetric flask. Then filter the digestate of the brown MnO2 precipitate from Container No. 1 through Whatman No. 40 filter
paper into the same 500-ml volumetric flask, and dilute to volume with water.
Mark this combined 500 ml dilute solution as analysis Sample No. A.2. Discard
the filters.
11.2.3 Container No.
5 (Absorbing Solution Blank) and No. 6 (HCl Rinse Blank).
11.2.3.1 Treat
Container No. 5 as Container No. 1 (as described in Section 11.2.2), except
substitute the filter blank filtrate from Container No. 4 for the sample
filtrate from Container No. 3, and mark as Sample A.1 Blank.
11.2.3.2 Treat
Container No. 6 as Container No. 1A, (as described in Section 11.2.2, except
substitute the filtrate from the digested blank MnO2 precipitate for the filtrate from the digested sample MnO2 precipitate, and mark as Sample No. A.2 Blank.
NOTE: When analyzing samples A.1 Blank and HCl A.2
Blank, always begin with 10 ml aliquots. This applies specifically to blank
samples.
11.3 Analysis.
Calibrate the
analytical equipment and develop a calibration curve as outlined in Section 10.0.
11.3.1 Mercury
Samples. Then repeat the procedure used to establish the calibration curve with
appropriately sized aliquots (1 to 10 ml) of the samples (from Sections 11.2.2
and 11.2.3) until two consecutive peak heights agree within 3 percent of their
average value. If the 10 ml sample is below the detectable limit, use a larger
aliquot (up to 20 ml), but decrease the volume of water added to the aeration
cell accordingly to prevent the solution volume from exceeding the capacity of
the aeration bottle. If the peak maximum of a 1.0 ml aliquot is off scale,
further dilute the original sample to bring the Hg concentration into the
calibration range of the spectrophotometer. If the Hg content of the absorbing
solution and filter blank is below the working range of the analytical method,
use zero for the blank.
11.3.2 Run a blank
and standard at least after every five samples to check the spectrophotometer
calibration; recalibrate as necessary.
11.3.3 Check for
Matrix Effects (optional). Same as Method
101, Section 11.3.3.
12.0 Data Analysis and Calculations.
NOTE: Carry out calculations, retaining at least one extra
decimal significant figure beyond that of the acquired data. Round off figures
only after the final calculation. Other forms of the equations may be used as
long as they give equivalent results.
12.1 Nomenclature.
C(fltr)Hg = Total ng of Hg in aliquot of KMnO4 filtrate and HNO3 digestion of filter
analyzed (aliquot of analysis Sample No. A.1).
C(fltr blk)Hg = Total ng of Hg in aliquot of KMnO4 blank and HNO3
digestion of blank filter analyzed
(aliquot of analysis Sample No. A.1 blank).
C(HCl blk)Hg = Total ng of Hg analyzed in aliquot of the
500-ml analysis Sample No. HCl A.2 blank.
C(HCl)Hg = Total ng of Hg analyzed in the aliquot from
the 500-ml analysis Sample No. HCl A.2.
DF = Dilution factor
for the HCl-digested Hg-containing solution, Analysis Sample No.
"HCl A.2."
DFblk = Dilution factor for the HCl-digested Hg
containing solution, Analysis Sample No. "HCl A.2 blank." (Refer to
sample No."Hcl A.2" dilution factor above.)
m(fltr)Hg = Total blank corrected µg of Hg in KMnO4 filtrate and HNO3 digestion of filter
sample.
m(HCl)Hg = Total blank corrected µg of Hg in HCl rinse
and HCl digestate of filter sample.
mHg = Total blank corrected Hg content in each
sample, µg.
S = Aliquot volume of
sample added to aeration cell, ml.
Sblk = Aliquot volume of blank added to aeration
cell, ml.
Vf(blk) = Solution volume of blank sample, 1000 ml for
samples diluted as described in Section11.2.2.
Vf(fltr) = Solution volume of original sample, normally
1000 ml for samples diluted as described in Section 11.2.2.
Vf(HCl) = Solution volume of original sample, 500 ml for
samples diluted as described in Section 11.2.1.
10-3 = Conversion factor, µg/ng.
12.2 Average Dry Gas
Meter Temperature and Average Orifice Pressure Drop, Dry Gas Volume, Volume of
Water Vapor Condensed, Moisture Content, Isokinetic Variation, and Stack Gas
Velocity and Volumetric Flow Rate. Same as Method
5, Sections 12.2 through 12.5, 12.11, and 12.12, respectively.
12.3 Total Mercury.
12.3.1 For each
source sample, correct the average maximum absorbance of the two consecutive
samples whose peak heights agree within 3 percent of their average for the
contribution of the blank. Use the calibration curve and these corrected
averages to determine the final total weight of Hg in ng in the aeration cell
for each source sample.
12.3.2 Correct for
any dilutions made to bring the sample into the working range of the
spectrophotometer.
![]()
NOTE: This dilution factor applies only to the
intermediate dilution steps, since the original sample volume [(Vf)HCL] of "HCl A.2" has been factored out
in the equation along with the sample aliquot (S). In Eq. 101A-1, the sample
aliquot, S, is introduced directly into the aeration cell for analysis
according to the procedure outlined in Section 11.3.1. A dilution factor is
required only if it is necessary to bring the sample into the analytical
instrument's calibration range.
NOTE: The maximum allowable blank subtraction for the
HCl is the lesser of the two following values: (1) the actual blank measured
value (analysis Sample No. HCl A.2 blank), or (2) 5% of the Hg content in the
combined Hcl rinse and digested sample (analysis Sample No. HCl A.2).
![]()
NOTE: The maximum allowable blank subtraction for the
HCl is the lesser of the two following values: (1) the actual blank measured
value (analysis Sample No. "A.1 blank"), or (2) 5% of the Hg content
in the filtrate (analysis Sample No. "A.1").
![]()
12.3 Mercury Emission
Rate. Same as Method 101, Section 12.3.
12.4 Determination of
Compliance. Same as Method 101, Section 12.4.
13.0 Method Performance.
13.1 Precision. Based
on eight paired-train tests, the intra-laboratory standard deviation was
estimated to be 4.8 µg/ml in the concentration range of 50 to 130 µg/m3.
13.2 Bias. [Reserved]
13.3 Range. After
initial dilution, the range of this method is 20 to 800 ng Hg/ml. The upper
limit can be extended by further dilution of the sample.
14.0 Pollution Prevention. [Reserved]
15.0 Waste Management. [Reserved]
16.0 References.
Same as Section 16.0 of Method 101, with the addition of the
following:
1. Mitchell, W.J., et.
al. Test Methods to Determine the
Mercury Emissions from Sludge Incineration Plants. U.S. Environmental
Protection Agency. Research Triangle Park, NC. Publication No.
EPA-600/4-79-058. September 1979.
2. Wilshire, Frank
W., et. al. Reliability
Study of the U.S. EPA's Method 101A - Determination of Particulate and Gaseous
Mercury Emissions. U.S. Environmental Protection Agency. Research Triangle
Park, NC. Report No. 600/D-31/219 AREAL 367, NTIS Acc No. PB91-233361.
3. Memorandum from
William J. Mitchell to Roger T. Shigehara discussing the potential safety
hazard in Section 7.2 of Method 101A. February 28, 1990.
17.0 Tables,
Diagrams, Flowcharts, And Validation Data. [Reserved]