METHOD 111 - DETERMINATION OF POLONIUM-210 EMISSIONS
FROM STATIONARY SOURCES
NOTE: This method does not include all of the
specifications (e.g.,
equipment and supplies) and procedures (e.g., sampling and analytical) essential to its
performance. Some material is incorporated by reference from methods in
appendix A to 40 CFR Part 60. Therefore, to obtain reliable results, persons
using this method should have a thorough knowledge of at least the following
additional test methods: Method 1, Method 2, Method 3, and Method 5.
5.2.1 Hydrochloric Acid
(HCl).
5.2.4 Perchloric Acid
(HClO4).
8.0 Sample Collection,
Preservation, Transport, and Storage. [Reserved]
9.2 Miscellaneous
Quality Control Measures.
10.0 Calibration and
Standardization.
10.1 Standardization of
Alpha Spectrometry System.
10.2 Preparation of
Standardized Solution of Polonium-209.
10.3 Standardization of
Internal Proportional Counter
11.1 Determination of
Procedure Background.
11.2 Determination of
Instrument Background.
11.3 Quality Assurance
Audit Samples.
11.6 Preparation of
Silver Disc for Spontaneous Electro-deposition.
12.0 Data Analysis and
Calculations.
13.0 Method
Performance. [Reserved]
14.0 Pollution
Prevention. [Reserved]
15.0 Waste Management.
[Reserved]
17.0 Tables, Diagrams,
Flowcharts, and Validation Data. [Reserved]
1.0 Scope and Application.
1.1 Analytes.
1.2 Applicability.
This method is
applicable for the determination of the polonium-210 content of particulate
matter samples collected from stationary source exhaust stacks, and for the use
of these data to calculate polonium-210 emissions from individual sources and
from all affected sources at a facility.
1.3 Data Quality Objectives.
Adherence to the
requirements of this method will enhance the quality of the data obtained from
air pollutant sampling methods.
2.0 Summary of Method.
A particulate
matter sample, collected according to Method 5, is analyzed for polonium-210
content: the polonium-210 in the sample is put in solution, deposited on a
metal disc, and the radioactive disintegration rate measured. Polonium in acid
solution spontaneously deposits on surfaces of metals that are more
electropositive than polonium. This principle is routinely used in the
radiochemical analysis of polonium-210. Data reduction procedures are provided,
allowing the calculation of polonium-210 emissions from individual sources and
from all affected sources at a facility, using data obtained from Methods 2 and
5 and from the analytical procedures herein.
3.0 Definitions. [Reserved]
4.0 Interferences. [Reserved]
5.0 Safety.
5.1 Disclaimer.
This method may
involve hazardous materials, operations, and equipment. This test method may
not address all of the safety problems associated with its use. It is the
responsibility of the user of this test method to establish appropriate safety
and health practices and determine the applicability of regulatory limitations
prior to performing this test method.
5.2 Corrosive Reagents.
The following
reagents are hazardous. Personal protective equipment and safe procedures are
useful in preventing chemical splashes. If contact occurs, immediately flush
with copious amounts of water at least 15 minutes. Remove clothing under shower
and decontaminate. Treat residual chemical burns as thermal burns.
5.2.1 Hydrochloric Acid (HCl).
Highly corrosive
liquid with toxic vapors. Vapors are highly irritating to eyes, skin, nose, and
lungs, causing severe damage. May cause bronchitis, pneumonia, or edema of
lungs. Exposure to concentrations of 0.13 to 0.2 percent can be lethal to
humans in a few minutes. Provide ventilation to limit exposure. Reacts with
metals, producing hydrogen gas.
5.2.2 Hydrofluoric Acid (HF).
Highly corrosive
to eyes, skin, nose, throat, and lungs. Reaction to exposure may be delayed by
24 hours or more. Provide ventilation to limit exposure.
5.2.3 Nitric Acid (HNO3).
Highly corrosive
to eyes, skin, nose, and lungs. Vapors cause bronchitis, pneumonia, or edema of
lungs. Reaction to inhalation may be delayed as long as 30 hours and still be
fatal. Provide ventilation to limit exposure. Strong oxidizer. Hazardous
reaction may occur with organic materials such as solvents.
5.2.4 Perchloric Acid (HClO4).
Corrosive to eyes,
skin, nose, and throat. Provide ventilation to limit exposure. Keep separate
from water and oxidizable materials to prevent vigorous evolution of heat,
spontaneous combustion, or explosion. Heat solutions containing HClO4 only in hoods specifically designed for HClO4.
6.0 Equipment and Supplies.
6.1 Alpha
Spectrometry System. Consisting of a multi-channel analyzer, biasing
electronics, silicon surface barrier detector, vacuum pump and chamber.
6.2 Constant
Temperature Bath at 85 ûC (185 ûF).
6.3 Polished
Silver Discs. 3.8 cm diameter, 0.4 mm thick with a small hole near the edge.
6.4 Glass Beakers.
400 ml, 150 ml.
6.5 Hot Plate,
Electric.
6.6 Fume Hood.
6.7 Teflon
Beakers, 150 ml.
6.8 Magnetic
Stirrer.
6.9 Stirring Bar.
6.10 Hooks. Plastic
or glass, to suspend plating discs.
6.11 Internal
Proportional Counter. For measuring alpha particles.
6.12 Nucleopore
Filter Membranes. 25 mm diameter, 0.2 micrometer pore size or equivalent.
6.13 Planchets.
Stainless steel, 32 mm diameter with 1.5 mm lip.
6.14 Transparent
Plastic Tape. 2.5 cm wide with adhesive on both sides.
6.15 Epoxy Spray
Enamel.
6.16 Suction
Filter Apparatus. For 25 mm diameter
filter.
6.17 Wash Bottles,
250 ml capacity.
6.18 Graduated Cylinder,
plastic, 25 ml capacity.
6.19 Volumetric
Flasks, 100 ml, 250 ml.
7.0 Reagents and Standards.
Unless otherwise
indicated, it is intended that all reagents conform to the specifications
established by the Committee on Analytical Reagents of the American Chemical
Society, where such specifications are available; otherwise, use the best
available grade.
7.1 Ascorbic Acid.
7.2 Ammonium
Hydroxide (NH4OH), 15 M.
7.3 Water.
Deionized distilled, to conform to ASTM D 1193-77 or 91 (incorporated by reference
- see ¤ 61.18), Type 3. Use in all dilutions requiring water.
7.4 Ethanol (C2H5OH),
95 percent.
7.5 Hydrochloric
Acid, 12 M.
7.6 Hydrochloric
Acid, 1 M. Dilute 83 ml of the 12 M HCl to 1 liter with distilled water.
7.7 Hydrofluoric
Acid, 29 M.
7.8 Hydrofluoric
Acid, 3 M. Dilute 52 ml of the 29 M HF to 500 ml with distilled water. Use a
plastic graduated cylinder and storage bottle.
7.9 Lanthanum
Carrier, 0.1 mg La+3/ml. Dissolve 0.078 gram lanthanum nitrate,
La(NO3)3á6H2O in 250 ml of 1 M HCl.
7.10 Nitric Acid,
16 M.
7.11 Perchloric
Acid, 12 M.
7.12 Polonium-209
Solution.
7.13 Silver
Cleaner. Any mild abrasive commercial silver cleaner.
7.14 Degreaser.
7.15 Standard Solution.
Standardized solution of an alpha-emitting actinide element, such as
plutonium-239 or americium-241.
8.0 Sample Collection, Preservation, Transport, and Storage. [Reserved]
9.0 Quality Control
9.1 General Requirement.
9.1.1 All analysts
using this method are required to demonstrate their ability to use the method
and to define their respective accuracy and precision criteria.
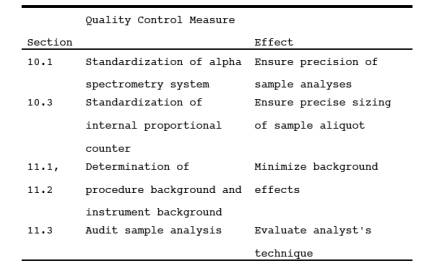
9.2 Miscellaneous Quality Control Measures.
10.0 Calibration and Standardization.
10.1 Standardization of Alpha Spectrometry System.
10.1.1 Add a
quantity of the actinide standard solution to a 100 ml volumetric flask so that
the final concentration when diluted to a volume of 100 ml will be
approximately 1 pCi/ml.
10.1.2 Add 10 ml
of 16 M HNO3 and dilute to 100 ml with water.
10.1.3 Add 20 ml
of 1 M HCl to each of six 150 ml beakers. Add 1.0 ml of lanthanum carrier, 0.1
mg lanthanum per ml, to the acid solution in each beaker.
10.1.4 Add 1.0 ml
of the 1 pCi/ml working solution (from Section 10.1.1) to each beaker. Add 5.0
ml of 3 M HF to each beaker.
10.1.5 Cover
beakers and allow solutions to stand for a minimum of 30 minutes. Filter the
contents of each beaker through a separate filter membrane using the suction
filter apparatus. After each filtration, wash the filter membrane with 10 ml of
distilled water and 5 ml of ethanol, and allow the filter membrane to air dry
on the filter apparatus.
10.1.6
Carefully remove the filter membrane and mount it, filtration side up, with
double-side tape on the inner surface of a planchet. Place planchet in an alpha
spectrometry system and count each planchet for 1000 minutes.
10.1.7 Calculate
the counting efficiency of the detector for each aliquot of the 1 pCi/ml
actinide working solution using Eq. 111-1 in Section
12.2.
10.1.8 Determine the average counting
efficiency of the detector, Ec, by calculating
the average of the six determinations.
10.2 Preparation of Standardized Solution of Polonium-209.
10.2.1 Add a quantity
of the Po-209 solution to a 100 ml volumetric flask so that the final
concentration when diluted to a 100 ml volume will be approximately 1 pCi/ml.
10.2.2 Follow the
procedures outlined in Sections 10.1.2 through 10.1.6, except substitute 1.0 ml
of polonium-209 tracer solution (Section 10.2.1) and 3.0 ml of 15 M ammonium
hydroxide for the 1 pCi/ml actinide working solution and the 3 M HF,
respectively.
10.2.3 Calculate
the activity of each aliquot of the polonium-209 tracer solution using Eq. 111-2 in Section 12.3.
10.2.4
Determine the average activity of the polonium-209 tracer solution, F, by
averaging the results of the six determinations.
10.3 Standardization of Internal Proportional Counter
10.3.1 Add a quantity
of the actinide standard solution to a 100 ml volumetric flask so that the
final concentration when diluted to a 100 ml volume will be approximately 100
pCi/ml.
10.3.2
Follow the procedures outlined in Sections 10.1.2 through 10.1.6, except
substitute the 100 pCi/ml actinide working solution for the 1 pCi/ml solution,
place the planchet in an internal proportional counter (instead of an alpha
spectrometry system), and count for 100 minutes (instead of 1000 minutes).
10.3.3 Calculate
the counting efficiency of the internal proportional counter for each aliquot
of the 100 pCi/ml actinide working solution using Eq.
111-3 in 12.4.
10.3.4 Determine
the average counting efficiency of the internal proportional counter, EI, by averaging the results of the six
determinations.
11.0 Analytical Procedure.
NOTE: Perform duplicate analyses of all samples,
including background counts, quality assurance audit samples, and Method 5
samples. Duplicate measurements are considered acceptable when the difference
between them is less than two standard deviations as described in EPA
600/4-77-001 or subsequent revisions.
11.1 Determination of Procedure Background.
Background counts
used in all equations are determined by performing the specific analysis
required using the analytical reagents only. All procedure background counts
and sample counts for the internal proportional counter should utilize a
counting time of 100 minutes; for the alpha spectrometry system, 1000 minutes.
These background counts should be performed no less frequently than once per 10
sample analyses.
11.2 Determination of Instrument Background.
Instrument
backgrounds of the internal proportional counter and the alpha spectrometry
system should be determined on a weekly basis. Instrument background should not
exceed procedure background. If this occurs, it may be due to a malfunction or
contamination, and should be corrected before use.
11.3 Quality Assurance Audit Samples.
An externally
prepared performance evaluation sample shall be analyzed no less frequently
than once per 10 sample analyses, and the results reported with the test
results.
11.4 Sample Preparation.
Treat the Method 5
samples [i.e., the glass
fiber filter (Container No. 1) and the acetone rinse (Container No. 2)] as
follows:
11.4.1 Container
No. 1. Transfer the filter and any loose particulate matter from the sample
container to a 150-ml Teflon beaker.
11.4.2 Container
No. 2. Note the level of liquid in the container, and confirm on the analysis
sheet whether leakage occurred during transport. If a noticeable amount of
leakage has occurred, either void the sample or use methods, subject to the
approval of the Administrator, to
correct the final results. Transfer the
contents to a 400-ml glass beaker. Add polonium-209 tracer solution to the
glass beaker in an amount approximately equal to the amount of polonium-210
expected in the total particulate sample. Record the activity of the tracer
solution added. Add 16 M nitric acid to the beaker to digest and loosen the
residue.
11.4.3 Transfer
the contents of the glass beaker to the Teflon beaker containing the glass
fiber filter. Rinse the glass beaker with 16 M HNO3. If necessary, reduce the volume in the
beaker by evaporation until all of the nitric acid HNO3 from the glass beaker has been transferred to
the Teflon beaker.
11.4.4 Add 30 ml
of 29 M HF to the Teflon beaker and evaporate to near dryness on a hot plate in
a properly operating hood.
NOTE: Do not allow the residue to go to dryness
and overheat; this will result in loss of polonium.
11.4.5 Repeat step
11.4.4 until the filter is dissolved.
11.4.6 Add 100 ml
of 16 M HNO3 to the residue in the Teflon beaker and evaporate
to near dryness.
NOTE: Do not allow the residue to go to dryness.
11.4.7 Add 50 ml
of 16 M HNO3 and 10 ml of 12 M perchloric acid to the
Teflon beaker and heat until dense fumes of perchloric acid are evolved.
11.4.8 Repeat steps
11.4.4 to 11.4.7 as necessary until sample is completely dissolved.
11.4.9 Add 10 ml
of 12 M HCl to the Teflon beaker and evaporate to dryness. Repeat additions and
evaporations several times.
11.4.10 Transfer
the sample to a 250-ml volumetric flask and dilute to volume with 3 M HCl.
11.5 Sample Screening.
To avoid
contamination of the alpha spectrometry system, check each sample as follows:
11.5.1 Add 20 ml
of 1 M HCl, 1 ml of the lanthanum carrier solution (0.1 mg La/ml), a 1 ml
aliquot of the sample solution from Section 11.4.10, and 3 ml of 15 M ammonium
hydroxide to a 250-ml beaker in the order listed. Allow this solution to stand
for a minimum of 30 minutes.
11.5.2 Filter the
solution through a filter membrane using the suction filter apparatus. Wash the
filter membrane with 10 ml of water and 5 ml of ethanol, and allow the filter
membrane to air dry on the filter apparatus.
11.5.3 Carefully
remove the filter membrane and mount it, filtration side up, with double-side
tape on the inner surface of a planchet. Place the planchet in an internal
proportional counter, and count for 100 minutes.
11.5.4 Calculate
the activity of the sample using Eq. 111-4 in Section
12.5.
11.5.5 Determine
the aliquot volume of the sample solution from Section 11.4.10 to be analyzed
for polonium-210 , such that the aliquot contains an activity between 1 and 4
picocuries. Use Eq. 111-5 in Section 12.6.
11.6 Preparation of Silver Disc for Spontaneous Electro-deposition.
11.6.1 Clean both
sides of the polished silver disc with silver cleaner and with degreaser.
11.6.2 Place disc
on absorbent paper and spray one side with epoxy spray enamel. This should be carried
out in a well-ventilated area, with the disc lying flat to keep paint on one
side only. Allow paint to dry for 24 hours before using disc for deposition.
11.7 Sample Analysis.
11.7.1 Add the
aliquot of sample solution from Section 11.4.10 to be analyzed for
polonium-210, the volume of which was determined in Section 11.5.5, to a
suitable 200-ml container to be placed in a constant temperature bath.
NOTE: Aliquot volume may require a larger
container.
11.7.2 If
necessary, bring the volume to 100 ml with 1 M HCl. If the aliquot volume
exceeds 100 ml, use total aliquot.
11.7.3 Add 200 mg
of ascorbic acid and heat solution to 85 ûC (185 ûF) in a constant temperature
bath.
11.7.4 Suspend a
silver disc in the heated solution using a glass or plastic rod with a hook
inserted through the hole in the disc. The disc should be totally immersed in
the solution, and the solution must be stirred constantly, at all times during
the plating operation. Maintain the disc in solution for 3 hours.
11.7.5 Remove the
silver disc, rinse with deionized distilled water, and allow to air dry at room
temperature.
11.7.6 Place the
disc, with deposition side (unpainted side) up, on a planchet and secure with
double-side plastic tape. Place the planchet with disc in alpha spectrometry
system and count for 1000 minutes.
12.0 Data Analysis and Calculations.
12.1 Nomenclature.
A = picocuries of
polonium-210 in the Method 5 sample (from Section 12.8).
AA = picocuries of actinide added.
AL = volume of sample aliquot used, in ml
(specified in Section 11.5.1 as 1 ml).
AS = aliquot to be analyzed, in ml.
BB = procedure background counts measured in
polonium-209 spectral region.
BT = polonium-209 tracer counts in sample.
CT = total counts in polonium-210 spectral
region.
D = decay
correction for time "t" (in days) from sample collection to sample
counting, given by: D=e-0.005t
EC = average counting efficiency of detector
(from Section 10.1.8), as counts per
disintegration.
ECi = counting efficiency of the detector for
aliquot i of the actinide working solution, counts per disintegration.
EI = average counting efficiency of the internal
proportional counter, as determined in Section 10.3.4, counts per
disintegration.
EIi = counting efficiency of the internal
proportional counter for aliquot i of the 100 pCi/ml actinide working solution,
counts per disintegration.
EY = the fraction of polonium-209 recovered on
the planchet (from Section 12.7).
F = average
activity of polonium-209 in sample (from Section
10.2.4), in pCi.
Fi = activity of aliquot i of the polonium-209
tracer solution, in pCi.
L = dilution
factor (unitless). This is the volume of sample solution prepared (specified as
250 ml in Section 11.1.10) divided by the volume of the aliquot of sample
solution analyzed for polonium-210 (from Section 11.7.1).
Mi = phosphorous rock processing rate of the
source being tested, during run i, Mg/hr.
Mk = phosphate rock processed annually by source
k, in Mg/yr.
n = number of
calciners at the elemental phosphorus plant.
P = total activity
of sample solution from Section 11.4.10, in pCi (see Eq. 111-4).
Qsd = volumetric flow rate of effluent stream, as
determined by Method 2, in dscm/hr.
S = annual
polonium-210 emissions from the entire facility, in curies/yr.
Vm(std) = volume of air sample, as determined by
Method 5, in dscm.
Xk = emission rate from source k, from Section 12.10, in curies/Mg.
10-12 = curies per picocurie.
2.22 =
disintegrations per minute per picocurie.
250 = volume of
solution from Section 11.4.10, in ml.
12.2
Counting Efficiency. Calculate the counting efficiency of the detector for each
aliquot of the 1 pCi/ml actinide working solution using Eq. 111-1.

where:
CB = background counts in same peak area as CS.
CS = gross counts in actinide peak.
T = counting time
in minutes, specified in Section 10.1.6 as 1000
minutes.
12.3
Polonium-209 Tracer Solution Activity. Calculate the activity of each aliquot
of the polonium-209 tracer solution using Eq. 111-2.

where:
CB = background counts in the 4.88 MeV region of
spectrum the in the counting time T.
CS = gross counts of polonium-209 in the 4.88
MeV region of the spectrum in the counting time T.
T = counting time,
specified in Section 10.1.6 as 1000 minutes.
12.4
Control Efficiency of Internal Proportional Counter. Calculate the counting
efficiency of the internal proportional counter for each aliquot of the 100
pCi/ml actinide working solution using Eq. 111-3.

where:
CB = gross counts of procedure background.
CS = gross counts of standard.
T = counting time
in minutes, specified in Section 10.3.2 as 100
minutes.
12.5
Calculate the activity of the sample using Eq. 111-4.

where:
CB = total counts of procedure background. (See Section 11.1).
CS = total counts of screening sample.
T = counting time
for sample and background (which must be equal), in minutes (specified in
Section 11.5.3 as 100 minutes).
12.6
Aliquot Volume. Determine the aliquot volume of the sample solution from
Section 11.4.10 to be analyzed for polonium-210 , such that the aliquot
contains an activity between 1 and 4 picocuries using Eq. 111-5.

12.7 Polonium-209
Recovery. Calculate the fraction of polonium-209 recovered on the planchet, EY, using Eq. 111-6.

where:
T = counting time,
specified in Section 11.1 as 1000 minutes.
12.8
Polonium-210 Activity. Calculate the activity of polonium-210 in the Method 5
sample (including glass fiber filter and acetone rinse) using Eq. 111-7.
![]()
where:
CB = procedure background counts in polonium-210
spectral region.
T = counting time,
specified in Section 11.1 as 1000 minutes for all alpha spectrometry sample and
background counts.
12.9 Emission Rate
from Each Stack.
12.9.1 For each test
run, i, on a stack, calculate the measured polonium-210 emission rate, RSi, using Eq. 111-8.

12.9.2 Determine
the average polonium-210 emission rate from the stack, RS, by taking the sum of the measured emission
rates for all runs, and dividing by the number of runs performed.
12.9.3 Repeat
steps 12.9.1 and 12.9.2 for each stack of each calciner.
12.10
Emission Rate from Each Source. Determine the total polonium-210 emission rate,
Xk, from each source, k, by taking the sum of
the average emission rates from all stacks to which the source exhausts.
12.11 Annual
Polonium-210 Emission Rate from Entire Facility. Determine the annual elemental
phosphorus plant emissions of polonium-210, S, using Eq. 111-9.

13.0 Method Performance. [Reserved]
14.0 Pollution Prevention. [Reserved]
15.0 Waste Management. [Reserved]
16.0 References.
1. Blanchard, R.L.
"Rapid Determination of Lead-210 and Polonium-210 in Environmental Samples
by Deposition on Nickel." Anal. Chem., 38:189, pp. 189-192. February 1966.
17.0 Tables, Diagrams, Flowcharts, and Validation Data. [Reserved]