Tip of the Week
Click HERE for Adobe version of this Tip
|
|
Measuring Gas
Velocity Pressure under Weird Conditions |
The following Tip comes courtesy of Doug
Rhoades.
Measuring
flow rates at various types of processing plants can present some interesting
problems. We’ll talk about a few of
these here. In order to understand these
problems, though, we need to review a few basics about velocity measurements.
The Bernoulli Equation
We
typically measure velocity with a pitot tube. This measurement is defined by a
familiar form of Bernoulli’s equation:
![]()
Our primary
direct measurement is the pitot differential pressure, or DP.
Rearranging Bernoulli’s equation shows us how DP relates to the other parameters we
measure:
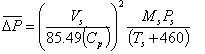
There are
three parameters here that can change (even during
a test run) in the equation that will have an influence on what we measure for DP:
- temperature (Ts)
- pressure (Ps)
- molecular weight (Ms)
Figure 1 shows the relationship between
gas temperature and expected velocity pressure.
DP is charted relative to the velocity pressure expected at
68°F, with all other variables held constant.
The figure shows that P
is an inverse function of temperature (for a given velocity).
Figure 2 shows the direct linear
relationship between expected DP and duct pressure. The velocity pressure has been normalized to
where a value of 1.0 is shown at a pressure of 29.921 in. Hg (one standard
atmosphere).
P is also linearly related to the gas molecular
weight. This is shown in Figure 3. The data are normalized to a standard
molecular weight of 28.84 lb/lb-mole (ambient air). If the gas molecular weight changes
drastically from this value, the measured P will be affected proportionally.
SCR Ducts
SCR ducts
are large, and the gas they transport is hot.
These factors combine to make velocity measurements at these locations a
challenge.
Most
ductwork is designed for an average gas velocity of around 60 ft/s under normal
operating conditions. For normal flue
gases, the gas density is anywhere between 0.04 and 0.08 lb/ft3. Under these normal gas densities, the
measurements can be straightforward.
However, when the gas density varies from this accustomed range,
difficulties can occur.
Before the
inlet of an SCR (i.e., economizer outlet conditions) the gas temperature can be
as high as 750°F. The gas density at
this temperature will be around 0.03 lb/ft3. This, combined with the large dimensions of
the duct, means that the gas velocity will fall to around 10 ft/s. This results in a P of around 0.05 in.W.C
close to the SCR (compared to around 0.5 in. W.C. at an ESP exit).
High Pressure Processes
Many
chemical and refinery processes operate at high pressures. Catalytic cracking
units are an example where the duct pressure is as high as 36 psig (103 in. Hg
absolute pressure). From Figure 2,
you would expect fairly high P
readings for a given flow rate (almost 3.4 times as high as at STP).
Hydrogen Streams
The
molecular weight of hydrogen is only 2.0 lb/lb-mole. We’re used to working with gases that are
about 10 times as dense as hydrogen (air and nitrogen). If a process gas is
hydrogen, you can expect very low Ps
for a given velocity. Digital recording
devices are strongly recommended when low velocity pressures are expected.
Dynamic Processes
For many
chemical processes, gas conditions may go through extreme variations over a
relatively short time frame. A typical
example involves a process reactor running without any flow until a valve is
switched and the duct pressure goes from zero (gauge) to 6 psi for about five
minutes as the reactor is purged with nitrogen. The duct pressure may then drop
to near zero gauge for the next hour as the liquid product is introduced to the
reactor. This occurs while purging the
headspace with nitrogen.
During this
time, the temperature will probably change as liquid (heat sink) is added to
the reactor. The pressure is also
dynamically changing during the first five minutes as the valve is switched.
