METHOD 106 -
DETERMINATION OF VINYL CHLORIDE EMISSIONS FROM STATIONARY SOURCES
6.4 Calibration and
Standardization.
7.2.4 Audit Cylinder
Standards.
8.0 Sample Collection,
Preservation, Storage, and Transport.
10.0 Calibration and
Standardization.
10.1 Preparation of
Vinyl Chloride Standard Gas Mixtures.
10.2 Determination of
Vinyl Chloride Retention Time.
10.3 Preparation of
Chromatograph Calibration Curve.
11.4 Determination of
Bag Water Vapor Content.
12.0 Calculations and
Data Analysis.
14.0 Pollution
Prevention. [Reserved]
15.0 Waste Management.
[Reserved]
17.0 Tables, Diagrams
Flowcharts, and Validation Data.
1.0 Scope and Application.
1.1 Analytes.

1.2 Applicability.
This method is
applicable for the determination of vinyl chloride emissions from ethylene
dichloride, vinyl chloride, and polyvinyl chloride manufacturing processes.
This method does not measure vinyl chloride contained in particulate matter.
1.3 Data Quality Objectives.
Adherence to the
requirements of this method will enhance the quality of the data obtained from
air pollutant sampling methods.
2.0 Summary of Method.
2.1 An integrated bag
sample of stack gas containing vinyl chloride is subjected to GC analysis using
a flame ionization detector (FID).
3.0 Definitions. [Reserved]
4.0 Interferences.
4.1 Resolution
interferences of vinyl chloride may be encountered on some sources. Therefore,
the chromatograph operator should select the column and operating parameters
best suited to the particular analysis requirements. The selection made is
subject to approval of the Administrator. Approval is automatic, provided that
confirming data are produced through an adequate supplemental analytical
technique, and that the data are available for review by the Administrator. An
example of this would be analysis with a different column or GC/mass
spectroscopy.
5.0 Safety.
5.1 Disclaimer. This
method may involve hazardous materials, operations, and equipment. This test
method may not address all of the safety problems associated with its use. It is
the responsibility of the user of this test method to establish appropriate
safety and health practices and determine the applicability of regulatory
limitations prior to performing this test method.
5.2 Toxic Analyte.
Care must be exercised to prevent exposure of sampling personnel to vinyl
chloride, which is a carcinogen.
6.0 Equipment and Supplies.
6.1 Sample Collection.
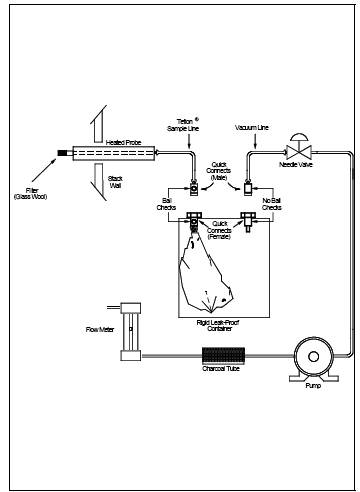
(See Figure 106-1) The sampling train consists of the following components:
6.1.1 Probe. Stainless
steel, borosilicate glass, Teflon tubing (as stack temperature permits), or
equivalent, equipped with a glass wool plug to remove particulate matter.
6.1.2 Sample Lines.
Teflon, 6.4-mm outside diameter, of sufficient length to connect probe to bag.
Use a new unused piece for each series of bag samples that constitutes an
emission test, and discard upon completion of the test.
6.1.3 Quick Connects.
Stainless steel, male (2) and female (2), with ball checks (one pair without),
located as shown in Figure 106-1.
6.1.4 Tedlar Bags.
50-to 100-liter capacity, to contain sample. Aluminized Mylar bags may be used
if the samples are analyzed within 24 hours of collection.
6.1.5 Bag Containers.
Rigid leak-proof containers for sample bags, with covering to protect contents
from sunlight.
6.1.6 Needle Valve.
To adjust sample flow rates.
6.1.7 Pump.
Leak-free, with minimum of 2-liter/min capacity.
6.1.8 Charcoal Tube.
To prevent admission of vinyl chloride and other organics to the atmosphere in
the vicinity of samplers.
6.1.9 Flowmeter. For
observing sampling flow rate; capable of measuring a flow range from 0.10 to
1.00 liter/min.
6.1.10 Connecting
Tubing. Teflon, 6.4-mm outside diameter, to assemble sampling train (Figure
106-1).
6.1.11 Tubing Fittings
and Connectors. Teflon or stainless steel, to assemble sampling training.
6.2 Sample Recovery.
Teflon tubing, 6.4-mm
outside diameter, to connect bag to GC sample loop. Use a new unused piece for
each series of bag samples that constitutes an emission test, and discard upon
conclusion of analysis of those bags.
6.3 Analysis.
The following
equipment is required:
6.3.1 Gas
Chromatograph. With FID potentiometric strip chart recorder and 1.0 to 5.0-ml
heated sampling loop in automatic sample valve. The chromatographic system
shall be capable of producing a response to 0.1-ppmv vinyl chloride that is at
least as great as the average noise level. (Response is measured from the
average value of the base line to the maximum of the wave form, while standard
operating conditions are in use.)
6.3.2 Chromatographic
Columns. Columns as listed below. Other columns may be used provided that the
precision and accuracy of the analysis of vinyl chloride standards are not
impaired and that information is available for review confirming that there is
adequate resolution of vinyl chloride peak. (Adequate resolution is defined as
an area overlap of not more than 10 percent of the vinyl chloride peak by an
interferent peak. Calculation of area overlap is explained in Procedure 1 of
appendix C to this part: "Determination of Adequate Chromatographic Peak
Resolution.")
6.3.2.1 Column A.
Stainless steel, 2.0 m by 3.2 mm, containing 80/100-mesh Chromasorb 102.
6.3.2.2 Column B. Stainless steel, 2.0 m by 3.2 mm, containing 20
percent GE SF-96 on 60/ip-mesh Chromasorb P AW; or stainless steel, 1.0 m by
3.2 mm containing 80/100-mesh Porapak T. Column B is required as a secondary
column if acetaldehyde is present. If used, column B is placed after column A.
The combined columns should be operated at 120 ¼C (250 ¼F).
6.3.3 Rate Meters
(2). Rotameter , or equivalent, 100-ml/min capacity, with flow control valves.
6.3.4 Gas Regulators.
For required gas cylinders.
6.3.5 Temperature
Sensor. Accurate to ±1 ¼C (± 2 ¼F), to measure temperature of heated sample
loop at time of sample injection.
6.3.6 Barometer.
Accurate to ±5 mm Hg, to measure atmospheric pressure around GC during sample
analysis.
6.3.7 Pump.
Leak-free, with minimum of 100-ml/min capacity.
6.3.8 Recorder. Strip
chart type, optionally equipped with either disc or electronic integrator.
6.3.9 Planimeter.
Optional, in place of disc or electronic integrator on recorder, to measure
chromatograph peak areas.
6.4 Calibration and Standardization.
6.4.1 Tubing. Teflon,
6.4-mm outside diameter, separate pieces marked for each calibration
concentration.
NOTE: The following items are required only if the
optional standard gas preparation procedures (Section
10.1) are followed.
6.4.2 Tedlar Bags.
Sixteen-inch-square size, with valve; separate bag marked for each calibration
concentration.
6.4.3 Syringes.
0.5-ml and 50-µl, gas tight, individually calibrated to dispense gaseous vinyl
chloride.
6.4.4 Dry Gas Meter
with Temperature and Pressure Gauges. Singer Model DTM-115 with 802 index, or
equivalent, to meter nitrogen in preparation of standard gas mixtures,
calibrated at the flow rate used to prepare standards.
7.0 Reagents and Standards.
7.1 Analysis.
The following
reagents are required for analysis.
7.1.1 Helium or
Nitrogen. Purity 99.9995 percent or greater, for chromatographic carrier gas.
7.1.2 Hydrogen.
Purity 99.9995 percent or greater.
7.1.3 Oxygen or Air.
Either oxygen (purity 99.99 percent or greater) or air (less than 0.1 ppmv
total hydrocarbon content), as required by detector.
7.2 Calibration.
Use one of the
following options: either Sections 7.2.1 and 7.2.2, or Section 7.2.3.
7.2.1 Vinyl Chloride.
Pure vinyl chloride
gas certified by the manufacturer to contain a minimum of 99.9 percent vinyl
chloride. If the gas manufacturer maintains a bulk cylinder supply of 99.9+
percent vinyl chloride, the certification analysis may have been performed on
this supply, rather than on each gas cylinder prepared from this bulk supply. The
date of gas cylinder preparation and the certified analysis must have been
affixed to the cylinder before shipment from the gas manufacturer to the buyer.
7.2.2 Nitrogen.
Same as described in
Section 7.1.1.
7.2.3 Cylinder Standards.
Gas mixture standards
(50-,10-, and 5 ppmv vinyl chloride) in nitrogen cylinders may be used to
directly prepare a chromatograph calibration curve as described in Section 10.3
if the following conditions are met: (a) The manufacturer certifies the gas
composition with an accuracy of ±3 percent or better. (b) The manufacturer
recommends a maximum shelf life over which the gas concentration does not
change by greater than ± 5 percent from the certified value. (c) The
manufacturer affixes the date of gas cylinder preparation, certified vinyl
chloride concentration, and recommended maximum shelf to the cylinder before
shipment to the buyer.
7.2.3.1 Cylinder
Standards Certification. The manufacturer shall certify the concentration of
vinyl chloride in nitrogen in each cylinder by (a) directly analyzing each
cylinder and (b) calibrating his analytical procedure on the day of cylinder
analysis. To calibrate his analytical procedure, the manufacturer shall use as
a minimum, a three point calibration curve. It is recommended that the
manufacturer maintain (1) a high concentration calibration standard (between 50
and 100 ppmv) to prepare his calibration curve by an appropriate dilution
technique and (2) a low-concentration calibration standard (between 5 and 10
ppmv) to verify the dilution technique used. If the difference between the
apparent concentration read from the calibration curve and the true
concentration assigned to the low-concentration calibration standard exceeds 5
percent of the true concentration, the manufacturer shall determine the source
of error and correct it, then repeat the three-point calibration.
7.2.3.2 Verification
of Manufacturer's Calibration Standards. Before using a standard, the
manufacturer shall verify each calibration standard (a) by comparing it to gas
mixtures prepared (with 99 mole percent vinyl chloride) in accordance with the
procedure described in Section 7.2.1 or (b) calibrating it against vinyl
chloride cylinder Standard Reference Materials (SRM's) prepared by the National
Institute of Standards and Technology, if such SRM's are available. The
agreement between the initially determined concentration value and the
verification concentration value must be ±5 percent. The manufacturer must
re-verify all calibration standards on a time interval consistent with the
shelf life of the cylinder standards sold.
7.2.4 Audit Cylinder Standards.
7.2.4.1 Gas mixture
standards with concentrations known only to the person supervising the analysis
of samples. The concentrations of the audit cylinders should be: one
low-concentration cylinder in the range of 5 to 20 ppmv vinyl chloride and one
high-concentration cylinder in the range of 20 to 50 ppmv. When available,
obtain audit samples from the appropriate EPA Regional Office or from the
responsible enforcement authority.
NOTE: The responsible enforcement agency should be
notified at least 30 days prior to the test date to allow sufficient time for
sample delivery.
7.2.4.2
Alternatively, audit cylinders obtained from a commercial gas manufacturer may
be used provided: (a) the gas meets the conditions described in Section 7.2.3,
(b) the gas manufacturer certifies the audit cylinder as described in Section
7.2.3.1, and (c) the gas manufacturer obtains an independent analysis of the audit
cylinders to verify this analysis. Independent analysis is defined here to mean
analysis performed by an individual different than the individual who performs
the gas manufacturer's analysis, while using calibration standards and analysis
equipment different from those used for the gas manufacturer's analysis.
Verification is complete and acceptable when the independent analysis
concentration is within 5 percent of the gas manufacturer's concentration.
8.0 Sample Collection, Preservation, Storage, and Transport.
NOTE: Performance of this method should not be
attempted by persons unfamiliar with the operation of a gas chromatograph (GC)
nor by those who are unfamiliar with source sampling, because knowledge beyond
the scope of this presentation is required.
8.1 Bag Leak-Check.
The following
leak-check procedure is recommended, but not required, prior to sample
collection. The post-test leak-check procedure is mandatory. Connect a water
manometer and pressurize the bag to 5 to 10 cm H2O (2
to 4 in. H2O). Allow to stand for 10 min. Any displacement
in the water manometer indicates a leak. Also, check the rigid container for
leaks in this manner.
NOTE: An alternative leak-check method is to
pressurize the bag to 5 to 10 cm H2O and allow
it to stand overnight. A deflated bag indicates a leak. For each sample bag in
its rigid container, place a rotameter in line between the bag and the pump
inlet. Evacuate the bag. Failure of the rotameter to register zero flow when
the bag appears to be empty indicates a leak.
8.2 Sample Collection.
Assemble the sample
train as shown in Figure 106-1. Join the quick connects as illustrated, and
determine that all connection between the bag and the probe are tight. Place
the end of the probe at the centroid of the stack and start the pump with the
needle valve adjusted to yield a flow that will fill over 50 percent of bag
volume in the specific sample period. After allowing sufficient time to purge
the line several times, change the vacuum line from the container to the bag
and evacuate the bag until the rotameter indicates no flow. Then reposition the
sample and vacuum lines and begin the actual sampling, keeping the rate
proportional to the stack velocity. At all times, direct the gas exiting the
rotameter away from sampling personnel. At the end of the sample period, shut
off the pump, disconnect the sample line from the bag, and disconnect the
vacuum line from the bag container. Protect the bag container from sunlight.
8.3 Sample Storage.
Keep the sample bags
out of direct sunlight. When at all possible, analysis is to be performed
within 24 hours, but in no case in excess of 72 hours of sample collection.
Aluminized Mylar bag samples must be analyzed within 24 hours.
8.4 Post-test Bag Leak-Check.
Subsequent to
recovery and analysis of the sample, leak-check the sample bag according to the
procedure outlined in Section 8.1.
9.0 Quality Control.
9.1 Miscellaneous
Quality Control.
9.2 Immediately after
the preparation of the calibration curve and prior to the sample analyses,
perform the analysis audit described in Appendix
C, Procedure 2: "Procedure for Field Auditing GC Analysis."
10.0 Calibration and Standardization.
NOTE: Maintain a laboratory log of all calibrations.
10.1 Preparation of Vinyl Chloride Standard Gas Mixtures.
(Optional
Procedure-delete if cylinder standards are used.) Evacuate a 16-inch square
Tedlar bag that has passed a leak-check (described in Section 8.1) and meter in
5.0 liters of nitrogen. While the bag is filling, use the 0.5-ml syringe to
inject 250 µl of 99.9+ percent vinyl chloride gas through the wall of the bag.
Upon withdrawing the syringe, immediately cover the resulting hole with a piece
of adhesive tape. The bag now contains a vinyl chloride concentration of 50
ppmv. In a like manner use the 50 µl syringe to prepare gas mixtures having 10-
and 5-ppmv vinyl chloride concentrations. Place each bag on a smooth surface
and alternately depress opposite sides of the bag 50 times to further mix the
gases. These gas mixture standards may be used for 10 days from the date of
preparation, after which time new gas mixtures must be prepared. (Caution:
Contamination may be a problem when a bag is reused if the new gas mixture
standard is a lower concentration than the previous gas mixture standard.)
10.2 Determination of Vinyl Chloride Retention Time.
(This section can be
performed simultaneously with Section 10.3.) Establish chromatograph conditions
identical with those in Section 11.3. Determine proper attenuator position.
Flush the sampling loop with helium or nitrogen and activate the sample valve.
Record the injection time, sample loop temperature, column temperature, carrier
gas flow rate, chart speed, and attenuator setting. Record peaks and detector
responses that occur in the absence of vinyl chloride. Maintain conditions with
the equipment plumbing arranged identically to Section 11.2, and flush the
sample loop for 30 seconds at the rate of 100 ml/min with one of the vinyl
chloride calibration mixtures. Then activate the sample valve. Record the
injection time. Select the peak that corresponds to vinyl chloride. Measure the
distance on the chart from the injection time to the time at which the peak
maximum occurs. This quantity divided by the chart speed is defined as the
retention time. Since other organics may be present in the sample, positive
identification of the vinyl chloride peak must be made.
10.3 Preparation of Chromatograph Calibration Curve.
Make a GC measurement
of each gas mixture standard (described in Section 7.2.3 or 10.1) using
conditions identical to those listed in Sections 11.2 and 11.3. Flush the
sampling loop for 30 seconds at the rate of 100 ml/min with one of the standard
mixtures, and activate the sample valve. Record the concentration of vinyl
chloride injected (Cc), attenuator setting, chart speed, peak area,
sample loop temperature, column temperature, carrier gas flow rate, and
retention time. Record the barometric pressure. Calculate Ac, the peak area multiplied by the attenuator setting. Repeat until
two consecutive injection areas are within 5 percent, then plot the average of
those two values versus Cc. When the other standard gas mixtures have been
similarly analyzed and plotted, draw a straight line through the points derived
by the least squares method. Perform calibration daily, or before and after the
analysis of each emission test set of bag samples, whichever is more frequent.
For each group of sample analyses, use the average of the two calibration
curves which bracket that group to determine the respective sample
concentrations. If the two calibration curves differ by more than 5 percent
from their mean value, then report the final results by both calibration
curves.
11.0 Analytical Procedure.
11.1 Audit Sample Analysis.
Immediately after the
preparation of the calibration curve and prior to the sample analyses, perform
the analysis audit described in Procedure 2 of appendix C to this part:
"Procedure for Field Auditing GC Analysis."
11.2 Sample Recovery.
With a new piece of
Teflon tubing identified for that bag, connect a bag inlet valve to the gas
chromatograph sample valve. Switch the valve to receive gas from the bag
through the sample loop. Arrange the equipment so the sample gas passes from
the sample valve to 100-ml/min rotameter with flow control valve followed by a
charcoal tube and a 1-in. H2O pressure gauge.
Maintain the sample flow either by a vacuum pump or container pressurization if
the collection bag remains in the rigid container. After sample loop purging is
ceased, allow the pressure gauge to return to zero before activating the gas
sampling valve.
11.3 Analysis.
11.3.1 Set the column
temperature to 100 ¼C (210 ¼F) and the detector temperature to 150 ¼C (300 ¼F).
When optimum hydrogen and oxygen (or air) flow rates have been determined,
verify and maintain these flow rates during all chromatography operations.
Using helium or nitrogen as the carrier gas, establish a flow rate in the range
consistent with the manufacturer's requirements for satisfactory detector
operation. A flow rate of approximately 40 ml/min should produce adequate
separations. Observe the base line periodically and determine that the noise
level has stabilized and that base line drift has ceased. Purge the sample loop
for 30 seconds at the rate of 100 ml/min, shut off flow, allow the sample loop
pressure to reach atmospheric pressure as indicated by the H2O manometer, then activate the sample valve. Record the injection
time (the position of the pen on the chart at the time of sample injection),
sample number, sample loop temperature, column temperature, carrier gas flow
rate, chart speed, and attenuator setting. Record the barometric pressure. From
the chart, note the peak having the retention time corresponding to vinyl
chloride as determined in Section 10.2. Measure the vinyl chloride peak area, Am, by use of a disc integrator, electronic integrator, or a
planimeter. Measure and record the peak heights, Hm. Record Am
and retention time. Repeat the
injection at least two times or until two consecutive values for the total area
of the vinyl chloride peak agree within 5 percent of their average. Use the
average value for these two total areas to compute the bag concentration.
11.3.2 Compare the
ratio of Hm to Am for the
vinyl chloride sample with the same ratio for the standard peak that is closest
in height. If these ratios differ by more than 10 percent, the vinyl chloride
peak may not be pure (possibly acetaldehyde is present) and the secondary
column should be employed (see Section 6.3.2.2).
11.4 Determination of Bag Water Vapor Content.
Measure the ambient
temperature and barometric pressure near the bag. From a water saturation vapor
pressure table, determine and record the water vapor content of the bag, Bwb, as a decimal figure. (Assume the relative humidity to be 100
percent unless a lesser value is known.)
12.0 Calculations and Data Analysis.
12.1 Nomenclature.

12.2 Sample Peak
Area. Determine the sample peak area, Ac, as
follows:
![]()
12.3 Vinyl Chloride
Concentration. From the calibration curves prepared in Section 10.3, determine
the average concentration value of vinyl chloride, Cc, that corresponds to Ac, the
sample peak area. Calculate the concentration of vinyl chloride in the bag, Cb, as follows:

13.0 Method Performance.
13.1 Analytical
Range. This method is designed for the 0.1 to 50 parts per million by volume
(ppmv) range. However, common gas chromatograph (GC) instruments are capable of
detecting 0.02 ppmv vinyl chloride. With proper calibration, the upper limit
may be extended as needed.
14.0 Pollution Prevention. [Reserved]
15.0 Waste Management. [Reserved]
16.0 References.
1. Brown D.W., E.W.
Loy, and M.H. Stephenson. Vinyl Chloride Monitoring Near the B. F. Goodrich
Chemical Company in Louisville, KY. Region IV, U.S. Environmental Protection
Agency, Surveillance and Analysis Division, Athens, GA. June 24, 1974.
2. G.D. Clayton and
Associates. Evaluation of a Collection and Analytical Procedure for Vinyl
Chloride in Air. U.S. Environmental Protection Agency, Research Triangle Park,
N.C. EPA Contract No. 68-02-1408, Task Order No. 2, EPA Report No. 75-VCL-1.
December 13, 1974.
3. Midwest Research
Institute. Standardization of Stationary Source Emission Method for Vinyl
Chloride. U.S. Environmental Protection Agency, Research Triangle Park, N.C.
Publication No. EPA-600/4-77-026. May 1977.
4. Scheil, G. and
M.C. Sharp. Collaborative Testing of EPA Method 106 (Vinyl Chloride) that Will
Provide for a Standardized Stationary Source Emission Measurement Method. U.S.
Environmental Protection Agency, Research Triangle Park, N.C. Publication No.
EPA 600/4-78-058. October 1978.
17.0 Tables, Diagrams Flowcharts, and Validation Data.
Figure
106-1. Integrated-bag sampling train.